User login
PANS and PANDAS – A step forward?
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Enterovirus D68 – An emerging threat to child health
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.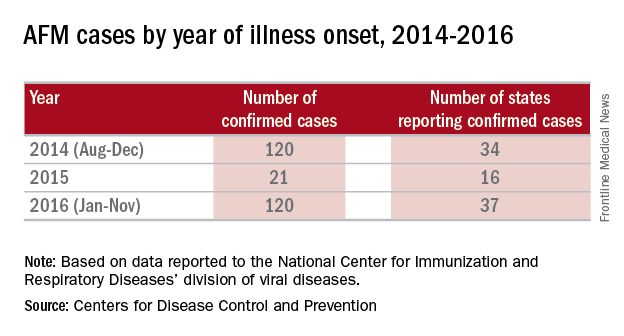
Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.
Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.
Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Influenza: A vaccine we love to hate
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
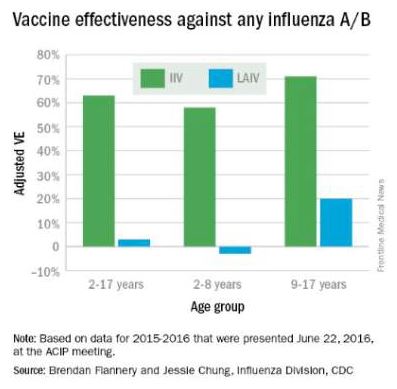
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.
ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
Appendicitis, antibiotics, and surgery: An evolving trilogy
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Protecting pregnant women, infants from infections
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.
However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
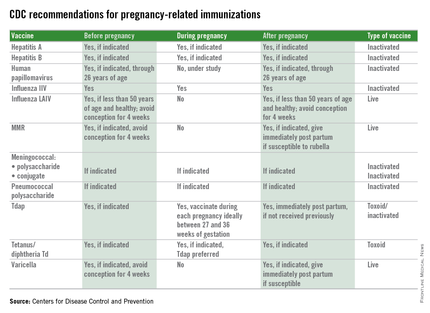
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at pdnews@frontlinemedcom.com.
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at pdnews@frontlinemedcom.com.
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at pdnews@frontlinemedcom.com.
Preventing recurrent staphylococcal skin and soft tissue infection
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at pdnews@frontlinemedcom.com.
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at pdnews@frontlinemedcom.com.
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at pdnews@frontlinemedcom.com.
ACIP and 2014 flu vaccine
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
Managing fever in the first month
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
New Paradigms in HIV Prevention
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
Universal SCIDS Screening Gaining Ground
Universal newborn screening for severe combined immunodeficiency syndrome is being adopted increasingly around the country.
Severe combined immunodeficiency (SCIDS) is a syndrome caused by a spectrum of mutations in genes necessary for the normal development and function of T cells, and therefore B cells. Identification of newborns with these mutations allows for early hematopoietic stem cell transplantation and a vastly improved prognosis. The evidence for an improved outcome for those identified early comes from studies comparing the outcome in first cases in a family with those in subsequent siblings who are diagnosed and transplanted earlier in life (Blood 2011;117:3243-6).
In May 2010, U.S. Secretary of Health and Human Services Kathleen Sebelius announced the addition of SCIDS to the national Recommended Uniform Screening Panel. Thus far, pilot programs for universal newborn SCIDS screening have been implemented in five states: California, Louisiana, Massachusetts, New York, and Wisconsin, and also in Puerto Rico. Another eight states, Colorado, Delaware, Florida, Iowa, Michigan, Minnesota, North Carolina, and Rhode Island, are prepared to implement SCIDS Newborn Screening Programs in the near future.
There are several types of SCIDS. Deficiency of the common gamma chain of the T-cell receptor is the most common, affecting nearly 45% of all cases. This mutation of the "cg" (for common gamma chain) gene on the X chromosome results in very low T-lymphocyte and NK-lymphocyte counts but the B-lymphocyte count is high. Only males have this type of SCIDS, but females may carry the gene.
The second-most common type, adenosine deaminase (ADA) deficiency, is caused by mutations in a gene that encodes the ADA enzyme, which is essential for the metabolic function of a variety of body cells, but especially T cells. The absence of ADA leads to accumulation of toxic metabolic by-products within lymphocytes that cause the cells to die. Infants with this type of SCIDS have the lowest total lymphocyte counts of all, and the T, B and NK-lymphocyte counts are all very low.
Another, less common SCIDS type is deficiency of the alpha chain of the IL-7 receptor, in which the infant has B and NK cells, but no T cells. However, the B cells don’t work because of the lack of T cells.
Other less frequent types include deficiency of Janus kinase 3 (JAK3), in which the infants have lab values similar to X-linked SCIDS (T, B+, NK), and deficiencies in the CD3 or CD45 chains that play key roles in T-cell function.
The classic symptoms of SCIDS are recurrent severe infections, chronic diarrhea, and failure to thrive. Persistent mucocutaneous candidiasis is a common early finding, and opportunistic infections with normally nonpathogenic organisms, such as Pneumocystis jiroveci (formerly carinii) also occur. Attenuated vaccine organisms, rotavirus and varicella, may cause severe or fatal infection. About 10 cases SCIDS presenting with persistent diarrhea following rotavirus vaccine have been reported (Vaccine 2010;28:6609-12).
The strategy for newborn SCIDS screening is based upon polymerase chain reaction quantification of T-cell receptor excision circles (TRECs) – small circles of DNA created in T-cells during their passage through the thymus – from dried blood spots (DBS) on newborn screening cards. A normal number, based on an evaluation of 5,766 Wisconsin newborns, is 708 TRECs/3.2 mm DBS (Public Health Rep. 2010;125 [Suppl 2]:88-95).
A low TREC number implies abnormal thymic function. Children with SCIDS are expected to have near zero (the screening cutoff is 25 TREC/uL). Children with an abnormally low TREC count on screening should be followed by more formal testing of T-cell number, including naive cell markers and T-cell function (mitogen stimulation) if sufficient T cells are present.
When notified about a positive test, the physician should immediately refer the infant to a center for lymphocyte subset analysis to confirm or refute the diagnosis. These infants should be "isolated" within the home by avoiding young children and any potentially contagious contacts. Prophylaxis for Pneumocystis jiroveci pneumonia could be given if a delay in further testing is likely, as well as replacement therapy with immune globulin might be indicated if assessment of humoral immunity demonstrates hypogammaglobulinemia.
Live attenuated vaccines normally given by 2 months of age, including rotavirus, bacille Calmette-Guérin, and oral poliovirus vaccine should NOT be administered to the infant, and measles-mumps-rubella and varicella vaccines should not be administered to the infant’s caregivers or family.
In Wisconsin, the first state to offer universal newborn SCIDS screening, 0.05% (35) newborns out of a total 71,000 had positive tests and underwent confirmatory testing. Another 0.17% (119) had initial unsatisfactory test results that required repeat. Of 194,056 newborns screened in Massachusetts during 2008-2011, 4 were identified with SCIDS. This gives us a rate of approximately 1 in 50,000 live births. Importantly, two of those four were identified prior to exhibiting any symptoms. The oldest, who had the JAK3 defect but was never ill prior to receiving a stem cell transplant, is now more than 10 months post transplant and is doing well. The second, who has been transplanted and is at home, had been hospitalized and "very ill" at the time of the screening but had not received a diagnosis (of deficiency of common gamma chain).
The latter two have also been transplanted. One was sick by 10 days of age with classic SCIDS symptoms (thrush, febrile, failure to thrive) and was admitted. The fourth was reportedly healthy at the time of the report but had been seen for a weight check because the mother was worried. She had previously lost two children who had died at 4 months of age of pneumonia.
Although early in the adoption of SCIDS screening, the Massachusetts’ experience demonstrates that infants with SCIDS can be identified early, often before signs and symptoms are present. However, most abnormal tests are not caused by SCIDS or other immune deficits, which may also be identified early as a result of TREC screening.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
*This story was updated December 2, 2011.
Universal newborn screening for severe combined immunodeficiency syndrome is being adopted increasingly around the country.
Severe combined immunodeficiency (SCIDS) is a syndrome caused by a spectrum of mutations in genes necessary for the normal development and function of T cells, and therefore B cells. Identification of newborns with these mutations allows for early hematopoietic stem cell transplantation and a vastly improved prognosis. The evidence for an improved outcome for those identified early comes from studies comparing the outcome in first cases in a family with those in subsequent siblings who are diagnosed and transplanted earlier in life (Blood 2011;117:3243-6).
In May 2010, U.S. Secretary of Health and Human Services Kathleen Sebelius announced the addition of SCIDS to the national Recommended Uniform Screening Panel. Thus far, pilot programs for universal newborn SCIDS screening have been implemented in five states: California, Louisiana, Massachusetts, New York, and Wisconsin, and also in Puerto Rico. Another eight states, Colorado, Delaware, Florida, Iowa, Michigan, Minnesota, North Carolina, and Rhode Island, are prepared to implement SCIDS Newborn Screening Programs in the near future.
There are several types of SCIDS. Deficiency of the common gamma chain of the T-cell receptor is the most common, affecting nearly 45% of all cases. This mutation of the "cg" (for common gamma chain) gene on the X chromosome results in very low T-lymphocyte and NK-lymphocyte counts but the B-lymphocyte count is high. Only males have this type of SCIDS, but females may carry the gene.
The second-most common type, adenosine deaminase (ADA) deficiency, is caused by mutations in a gene that encodes the ADA enzyme, which is essential for the metabolic function of a variety of body cells, but especially T cells. The absence of ADA leads to accumulation of toxic metabolic by-products within lymphocytes that cause the cells to die. Infants with this type of SCIDS have the lowest total lymphocyte counts of all, and the T, B and NK-lymphocyte counts are all very low.
Another, less common SCIDS type is deficiency of the alpha chain of the IL-7 receptor, in which the infant has B and NK cells, but no T cells. However, the B cells don’t work because of the lack of T cells.
Other less frequent types include deficiency of Janus kinase 3 (JAK3), in which the infants have lab values similar to X-linked SCIDS (T, B+, NK), and deficiencies in the CD3 or CD45 chains that play key roles in T-cell function.
The classic symptoms of SCIDS are recurrent severe infections, chronic diarrhea, and failure to thrive. Persistent mucocutaneous candidiasis is a common early finding, and opportunistic infections with normally nonpathogenic organisms, such as Pneumocystis jiroveci (formerly carinii) also occur. Attenuated vaccine organisms, rotavirus and varicella, may cause severe or fatal infection. About 10 cases SCIDS presenting with persistent diarrhea following rotavirus vaccine have been reported (Vaccine 2010;28:6609-12).
The strategy for newborn SCIDS screening is based upon polymerase chain reaction quantification of T-cell receptor excision circles (TRECs) – small circles of DNA created in T-cells during their passage through the thymus – from dried blood spots (DBS) on newborn screening cards. A normal number, based on an evaluation of 5,766 Wisconsin newborns, is 708 TRECs/3.2 mm DBS (Public Health Rep. 2010;125 [Suppl 2]:88-95).
A low TREC number implies abnormal thymic function. Children with SCIDS are expected to have near zero (the screening cutoff is 25 TREC/uL). Children with an abnormally low TREC count on screening should be followed by more formal testing of T-cell number, including naive cell markers and T-cell function (mitogen stimulation) if sufficient T cells are present.
When notified about a positive test, the physician should immediately refer the infant to a center for lymphocyte subset analysis to confirm or refute the diagnosis. These infants should be "isolated" within the home by avoiding young children and any potentially contagious contacts. Prophylaxis for Pneumocystis jiroveci pneumonia could be given if a delay in further testing is likely, as well as replacement therapy with immune globulin might be indicated if assessment of humoral immunity demonstrates hypogammaglobulinemia.
Live attenuated vaccines normally given by 2 months of age, including rotavirus, bacille Calmette-Guérin, and oral poliovirus vaccine should NOT be administered to the infant, and measles-mumps-rubella and varicella vaccines should not be administered to the infant’s caregivers or family.
In Wisconsin, the first state to offer universal newborn SCIDS screening, 0.05% (35) newborns out of a total 71,000 had positive tests and underwent confirmatory testing. Another 0.17% (119) had initial unsatisfactory test results that required repeat. Of 194,056 newborns screened in Massachusetts during 2008-2011, 4 were identified with SCIDS. This gives us a rate of approximately 1 in 50,000 live births. Importantly, two of those four were identified prior to exhibiting any symptoms. The oldest, who had the JAK3 defect but was never ill prior to receiving a stem cell transplant, is now more than 10 months post transplant and is doing well. The second, who has been transplanted and is at home, had been hospitalized and "very ill" at the time of the screening but had not received a diagnosis (of deficiency of common gamma chain).
The latter two have also been transplanted. One was sick by 10 days of age with classic SCIDS symptoms (thrush, febrile, failure to thrive) and was admitted. The fourth was reportedly healthy at the time of the report but had been seen for a weight check because the mother was worried. She had previously lost two children who had died at 4 months of age of pneumonia.
Although early in the adoption of SCIDS screening, the Massachusetts’ experience demonstrates that infants with SCIDS can be identified early, often before signs and symptoms are present. However, most abnormal tests are not caused by SCIDS or other immune deficits, which may also be identified early as a result of TREC screening.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
*This story was updated December 2, 2011.
Universal newborn screening for severe combined immunodeficiency syndrome is being adopted increasingly around the country.
Severe combined immunodeficiency (SCIDS) is a syndrome caused by a spectrum of mutations in genes necessary for the normal development and function of T cells, and therefore B cells. Identification of newborns with these mutations allows for early hematopoietic stem cell transplantation and a vastly improved prognosis. The evidence for an improved outcome for those identified early comes from studies comparing the outcome in first cases in a family with those in subsequent siblings who are diagnosed and transplanted earlier in life (Blood 2011;117:3243-6).
In May 2010, U.S. Secretary of Health and Human Services Kathleen Sebelius announced the addition of SCIDS to the national Recommended Uniform Screening Panel. Thus far, pilot programs for universal newborn SCIDS screening have been implemented in five states: California, Louisiana, Massachusetts, New York, and Wisconsin, and also in Puerto Rico. Another eight states, Colorado, Delaware, Florida, Iowa, Michigan, Minnesota, North Carolina, and Rhode Island, are prepared to implement SCIDS Newborn Screening Programs in the near future.
There are several types of SCIDS. Deficiency of the common gamma chain of the T-cell receptor is the most common, affecting nearly 45% of all cases. This mutation of the "cg" (for common gamma chain) gene on the X chromosome results in very low T-lymphocyte and NK-lymphocyte counts but the B-lymphocyte count is high. Only males have this type of SCIDS, but females may carry the gene.
The second-most common type, adenosine deaminase (ADA) deficiency, is caused by mutations in a gene that encodes the ADA enzyme, which is essential for the metabolic function of a variety of body cells, but especially T cells. The absence of ADA leads to accumulation of toxic metabolic by-products within lymphocytes that cause the cells to die. Infants with this type of SCIDS have the lowest total lymphocyte counts of all, and the T, B and NK-lymphocyte counts are all very low.
Another, less common SCIDS type is deficiency of the alpha chain of the IL-7 receptor, in which the infant has B and NK cells, but no T cells. However, the B cells don’t work because of the lack of T cells.
Other less frequent types include deficiency of Janus kinase 3 (JAK3), in which the infants have lab values similar to X-linked SCIDS (T, B+, NK), and deficiencies in the CD3 or CD45 chains that play key roles in T-cell function.
The classic symptoms of SCIDS are recurrent severe infections, chronic diarrhea, and failure to thrive. Persistent mucocutaneous candidiasis is a common early finding, and opportunistic infections with normally nonpathogenic organisms, such as Pneumocystis jiroveci (formerly carinii) also occur. Attenuated vaccine organisms, rotavirus and varicella, may cause severe or fatal infection. About 10 cases SCIDS presenting with persistent diarrhea following rotavirus vaccine have been reported (Vaccine 2010;28:6609-12).
The strategy for newborn SCIDS screening is based upon polymerase chain reaction quantification of T-cell receptor excision circles (TRECs) – small circles of DNA created in T-cells during their passage through the thymus – from dried blood spots (DBS) on newborn screening cards. A normal number, based on an evaluation of 5,766 Wisconsin newborns, is 708 TRECs/3.2 mm DBS (Public Health Rep. 2010;125 [Suppl 2]:88-95).
A low TREC number implies abnormal thymic function. Children with SCIDS are expected to have near zero (the screening cutoff is 25 TREC/uL). Children with an abnormally low TREC count on screening should be followed by more formal testing of T-cell number, including naive cell markers and T-cell function (mitogen stimulation) if sufficient T cells are present.
When notified about a positive test, the physician should immediately refer the infant to a center for lymphocyte subset analysis to confirm or refute the diagnosis. These infants should be "isolated" within the home by avoiding young children and any potentially contagious contacts. Prophylaxis for Pneumocystis jiroveci pneumonia could be given if a delay in further testing is likely, as well as replacement therapy with immune globulin might be indicated if assessment of humoral immunity demonstrates hypogammaglobulinemia.
Live attenuated vaccines normally given by 2 months of age, including rotavirus, bacille Calmette-Guérin, and oral poliovirus vaccine should NOT be administered to the infant, and measles-mumps-rubella and varicella vaccines should not be administered to the infant’s caregivers or family.
In Wisconsin, the first state to offer universal newborn SCIDS screening, 0.05% (35) newborns out of a total 71,000 had positive tests and underwent confirmatory testing. Another 0.17% (119) had initial unsatisfactory test results that required repeat. Of 194,056 newborns screened in Massachusetts during 2008-2011, 4 were identified with SCIDS. This gives us a rate of approximately 1 in 50,000 live births. Importantly, two of those four were identified prior to exhibiting any symptoms. The oldest, who had the JAK3 defect but was never ill prior to receiving a stem cell transplant, is now more than 10 months post transplant and is doing well. The second, who has been transplanted and is at home, had been hospitalized and "very ill" at the time of the screening but had not received a diagnosis (of deficiency of common gamma chain).
The latter two have also been transplanted. One was sick by 10 days of age with classic SCIDS symptoms (thrush, febrile, failure to thrive) and was admitted. The fourth was reportedly healthy at the time of the report but had been seen for a weight check because the mother was worried. She had previously lost two children who had died at 4 months of age of pneumonia.
Although early in the adoption of SCIDS screening, the Massachusetts’ experience demonstrates that infants with SCIDS can be identified early, often before signs and symptoms are present. However, most abnormal tests are not caused by SCIDS or other immune deficits, which may also be identified early as a result of TREC screening.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
*This story was updated December 2, 2011.