Article
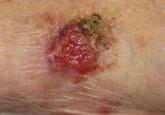
Cutaneous Adenosquamous Carcinoma: A Rare Neoplasm With Biphasic Differentiation
- Author:
- Vikas Patel, MD
- Stephen M. Squires, MD
- Deede Y. Liu, MD
Cutaneous adenosquamous carcinoma (cASC) is an extremely rare malignant neoplasm. Due to its disputed pathophysiology, ambiguity of presentation,...
