Article
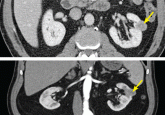
Small renal masses: Toward more rational treatment
- Author:
- Anil A. Thomas, MD
- Steven C. Campbell, MD, PhD
Although surgical removal of the whole kidney has long been the most common treatment, nephron-sparing approaches are now favored.
Article
Screening for urologic malignancies in primary care: Pros, cons, and recommendations
- Author:
- Andrew J. Stephenson, MD
- Louis Kuritzky, MD
- Steven C. Campbell, MD, PhD
