User login
Clinical presentation and imaging of bone and soft-tissue sarcomas
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
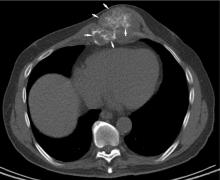
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
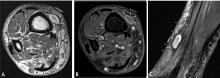
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
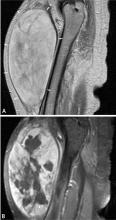
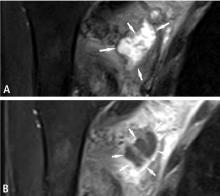
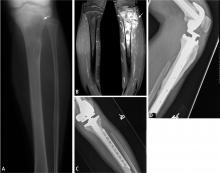
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
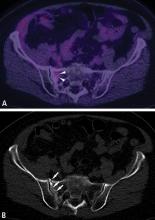
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
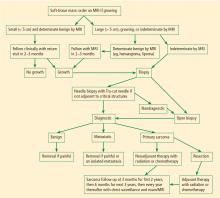
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
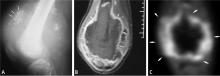
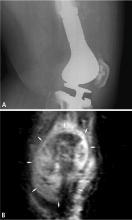
BIOPSY
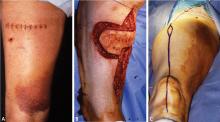
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
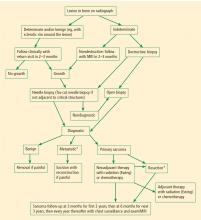
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.