User login
Etanercept-Induced Squamous Proliferations in a Patient With Porokeratosis
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
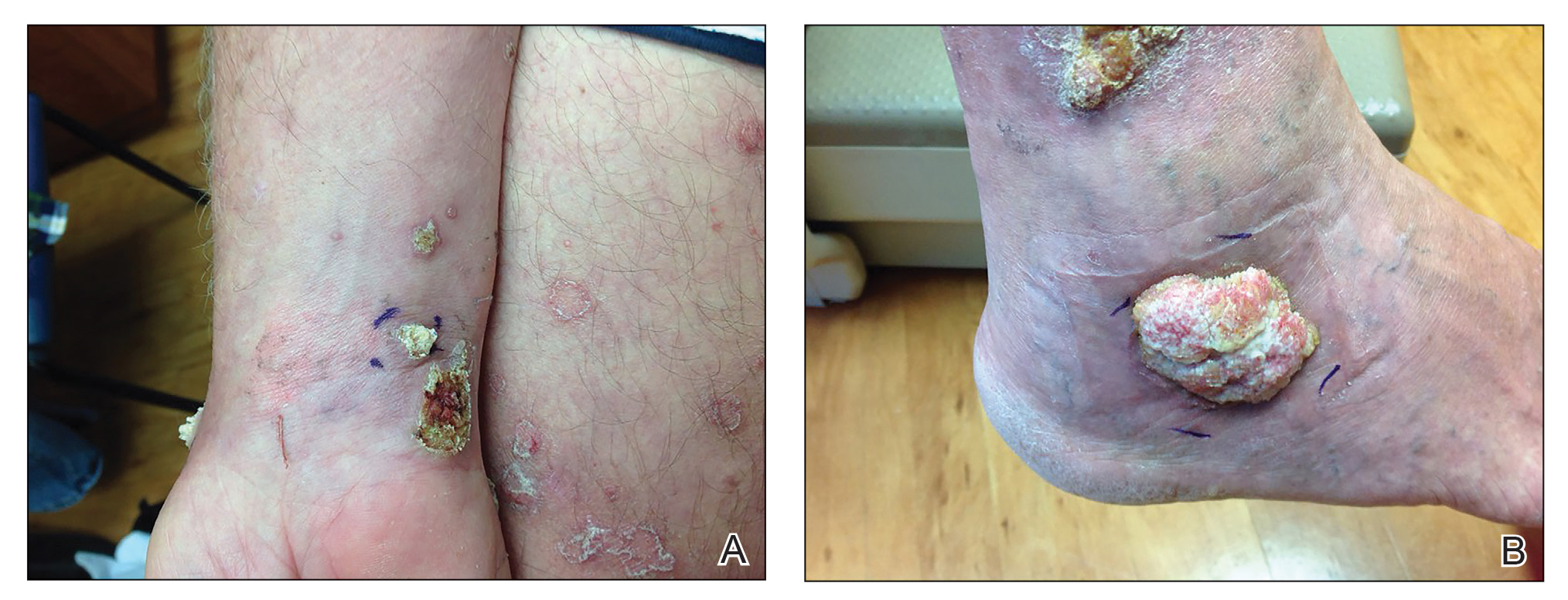
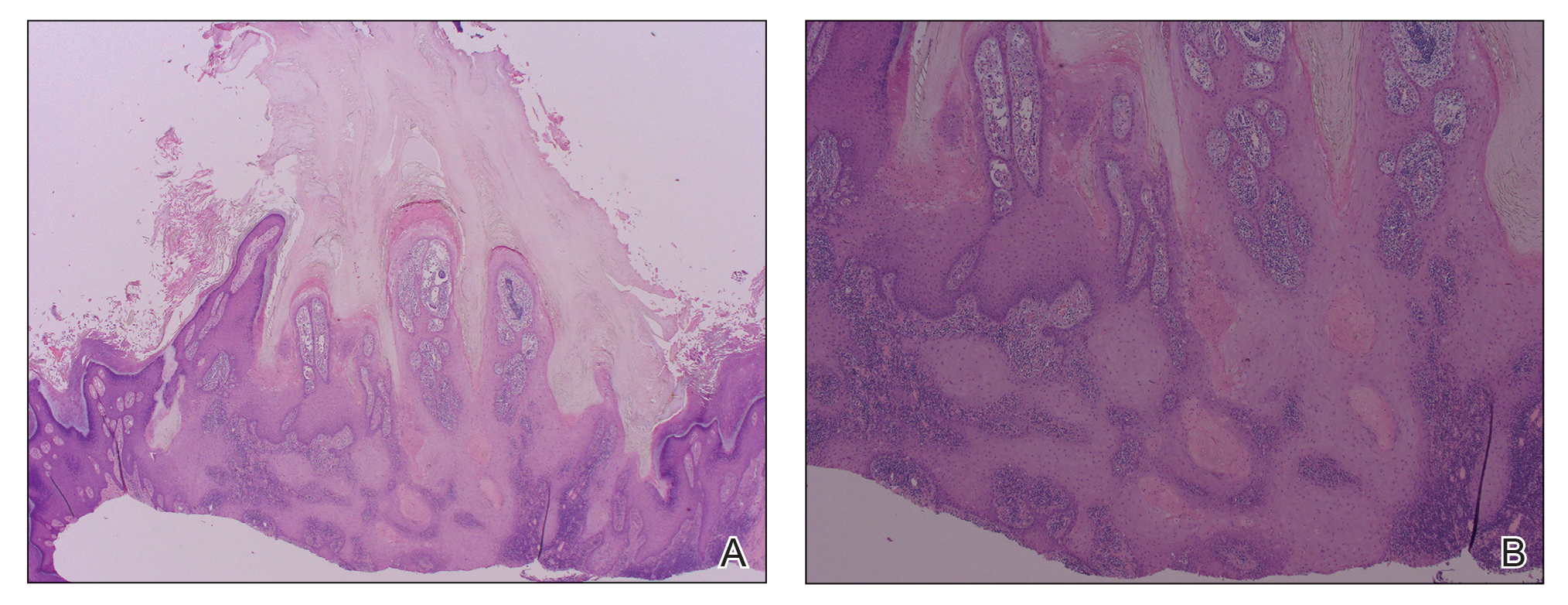
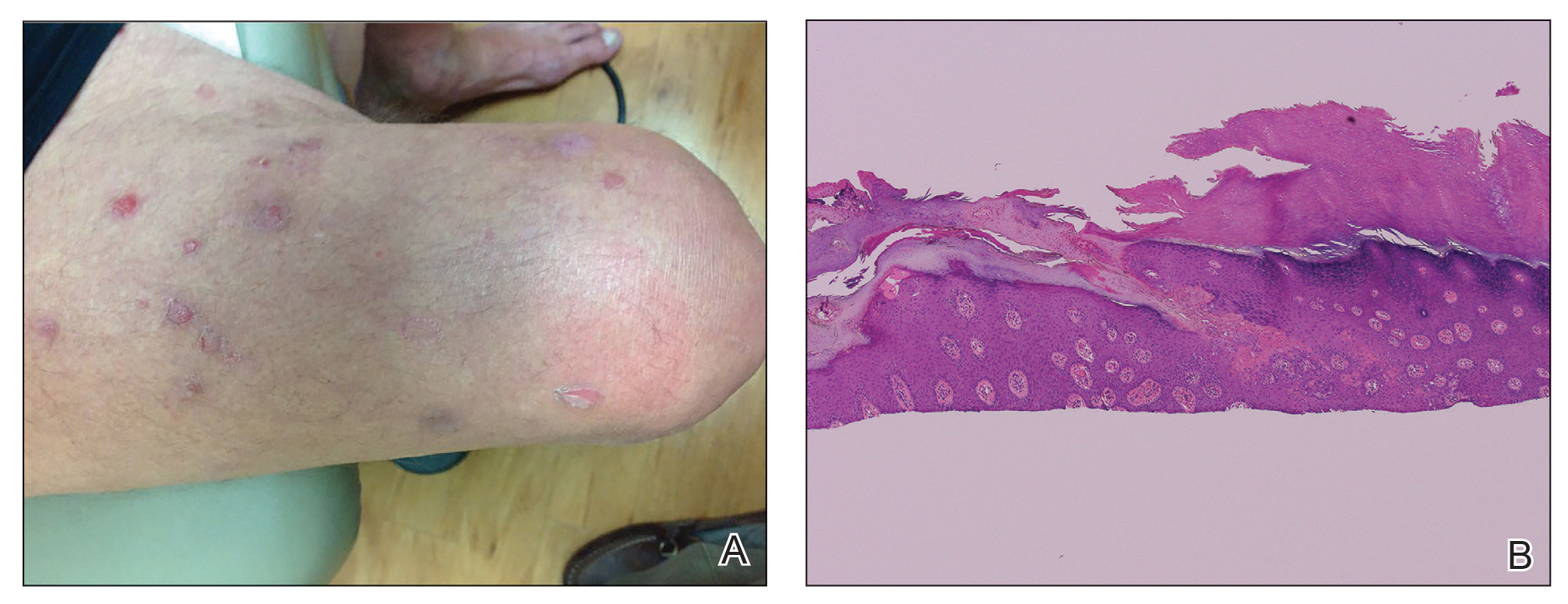
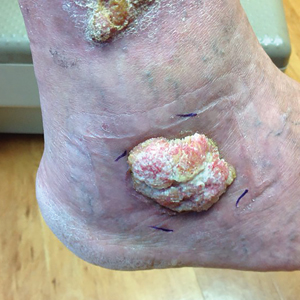
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
Practice Points
- The use of biologics, particularly tumor necrosis factor α blockers, rarely are reported to induce skin cancer.
- Squamous cell carcinoma in the setting of biologic treatment would warrant a change of systemic medication.
Tender Edematous Nodules on the Hand
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
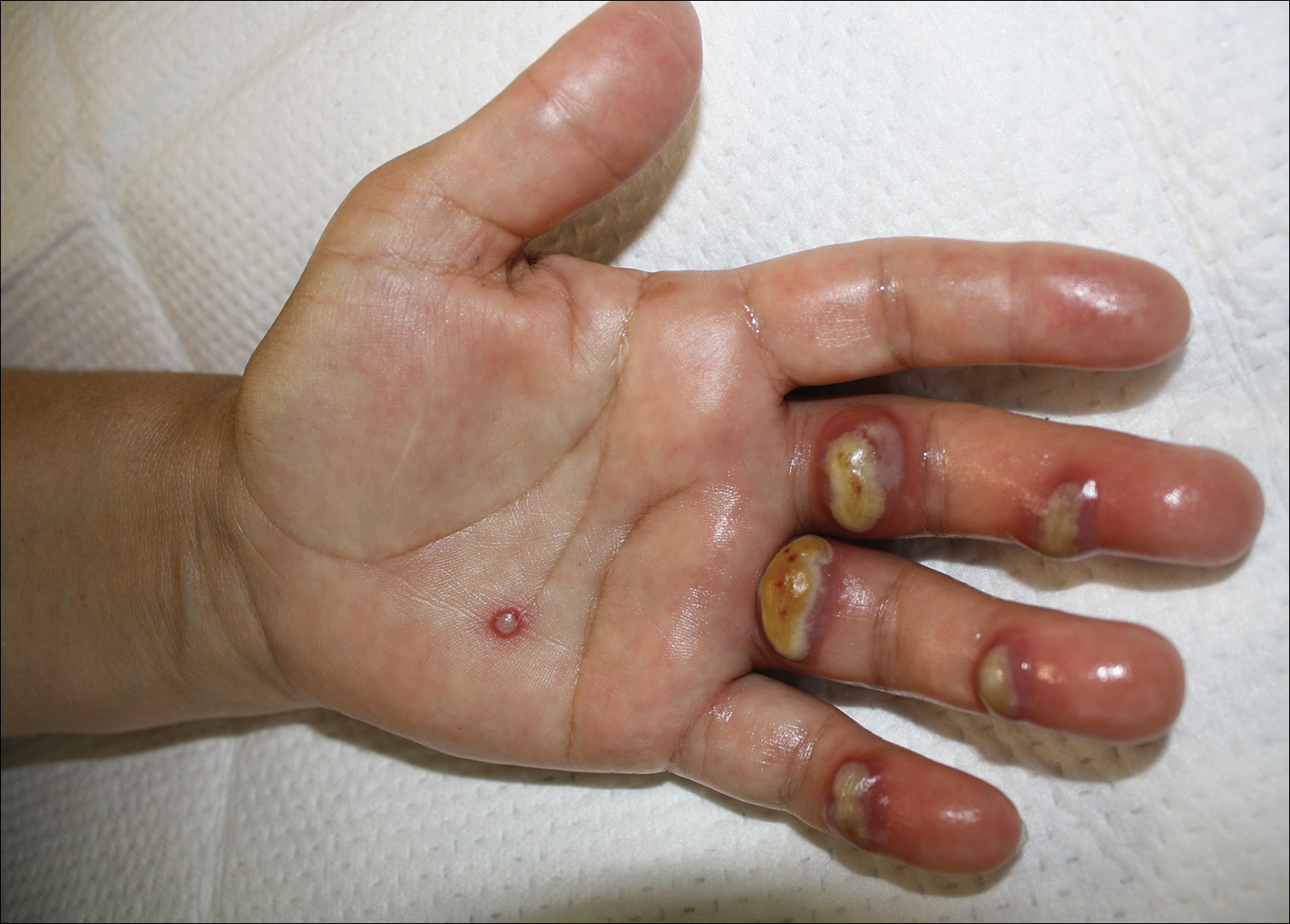
A 57-year-old woman presented to the emergency department (ED) for evaluation of a rash on the left hand of 2 weeks' duration. She described pinpoint red lesions on the left palm, as well as the third, fourth, and fifth fingers, which gradually enlarged and became painful. She denied any specific trauma but recalled cutting her hand on a piece of metal in the ground prior to the onset of the rash. She worked on a farm and bottle-fed sheep and chickens. Physical examination revealed tender edematous nodules with central gray pustules, and the left axillary lymph node was enlarged and tender. Ulceration was not appreciated. Various antibiotics including cephalexin, trimethoprim-sulfamethoxazole, and clindamycin were prescribed during prior ED visits, but she reported no improvement with these medications. She remained afebrile throughout the course of the hand rash, and laboratory workup was consistently unremarkable. Two sets of herpes simplex virus cultures from the ED visits showed no growth, and a hand radiograph also was normal. Medical history included coronary artery disease, myocardial infarction, mitral regurgitation, and hyperlipidemia.