User login
Severe Acute Systemic Reaction After the First Injections of Ixekizumab
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
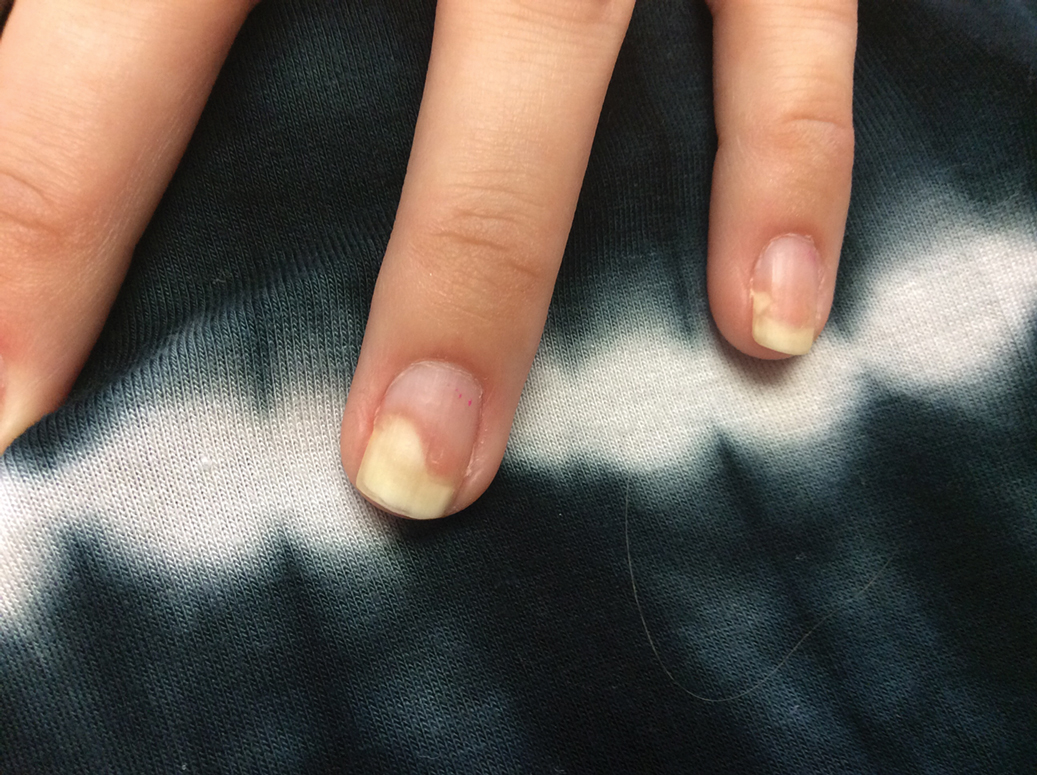
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
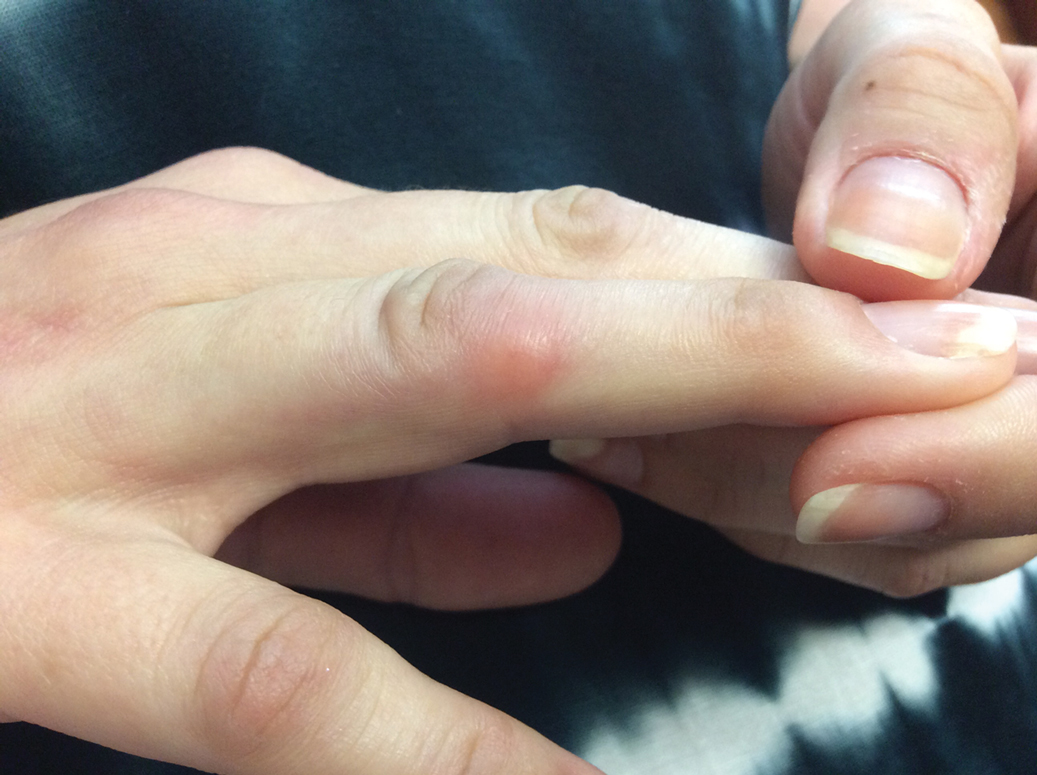
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Practice Points
- Psoriasis is an autoimmune disorder with a predominance of CD4+ and CD8+ T cells that release cytokines, such as tumor necrosis factor 11α and interleukins, which promote inflammation in the skin and joints and is associated with systemic inflammation predisposing patients to cardiovascular disease.
- Common adverse effects of most biologic medications for psoriasis include injection-site pain and rash, fever, malaise, back pain, urticaria and flushing, edema, dyspnea, and nausea.
- Ixekizumab is a humanized IL-17A antagonist intended for adults with moderate to severe psoriasis. Certain rare side effects specific to ixekizumab include inflammatory bowel disease, thrombocytopenia, severe injection-site reactions, and candidiasis.
- Acute onycholysis and acute exacerbation of arthritis/dactylitis are rare side effects of ixekizumab therapy.
Etanercept-Induced Squamous Proliferations in a Patient With Porokeratosis
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
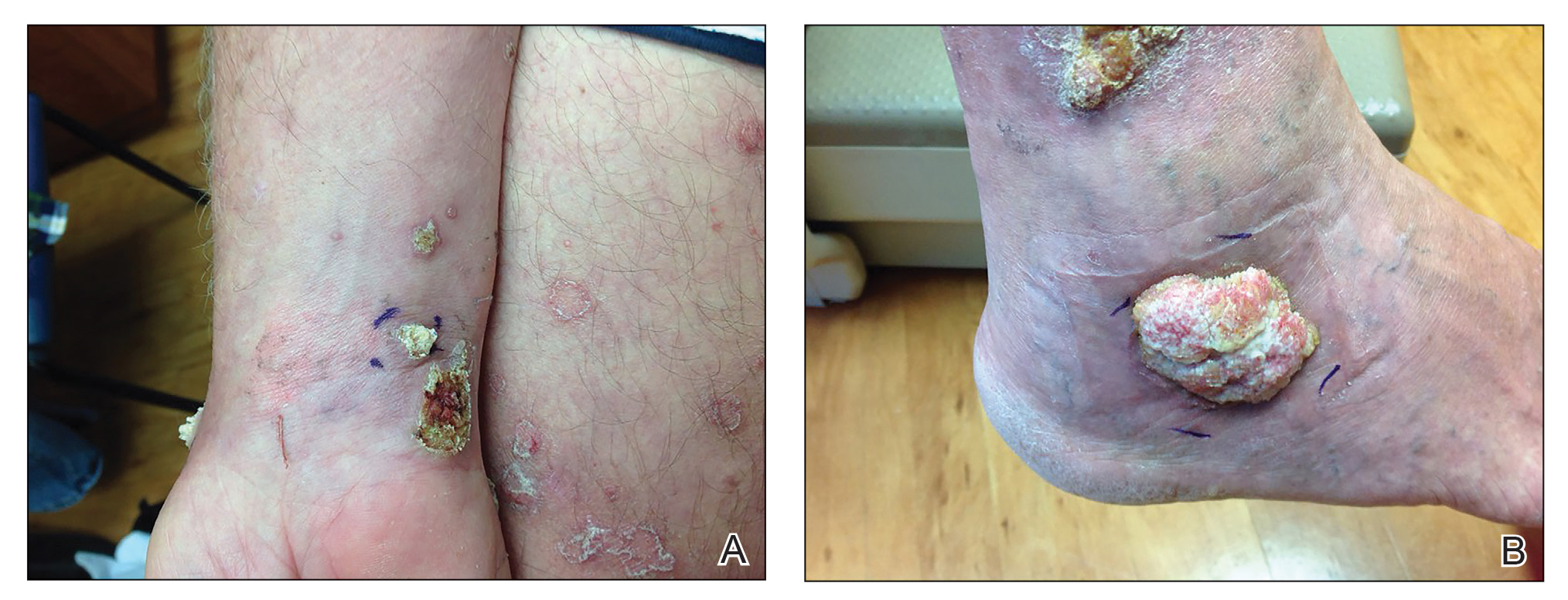
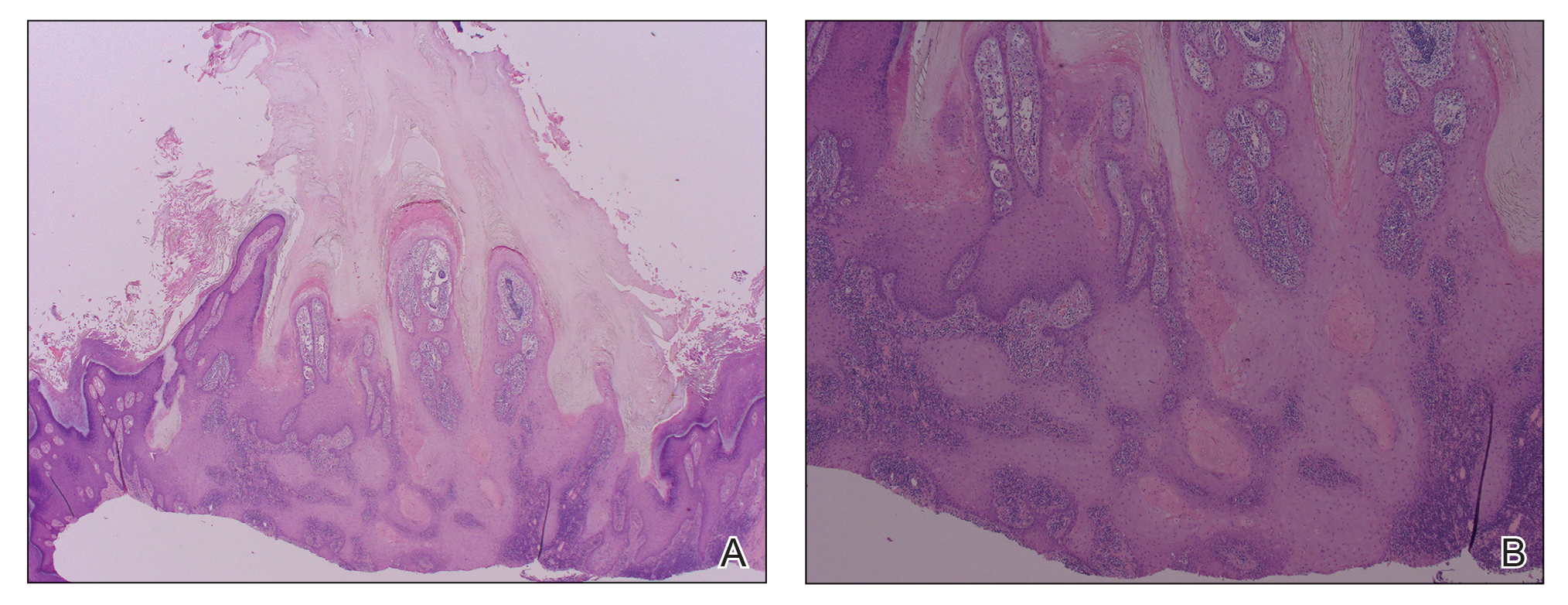
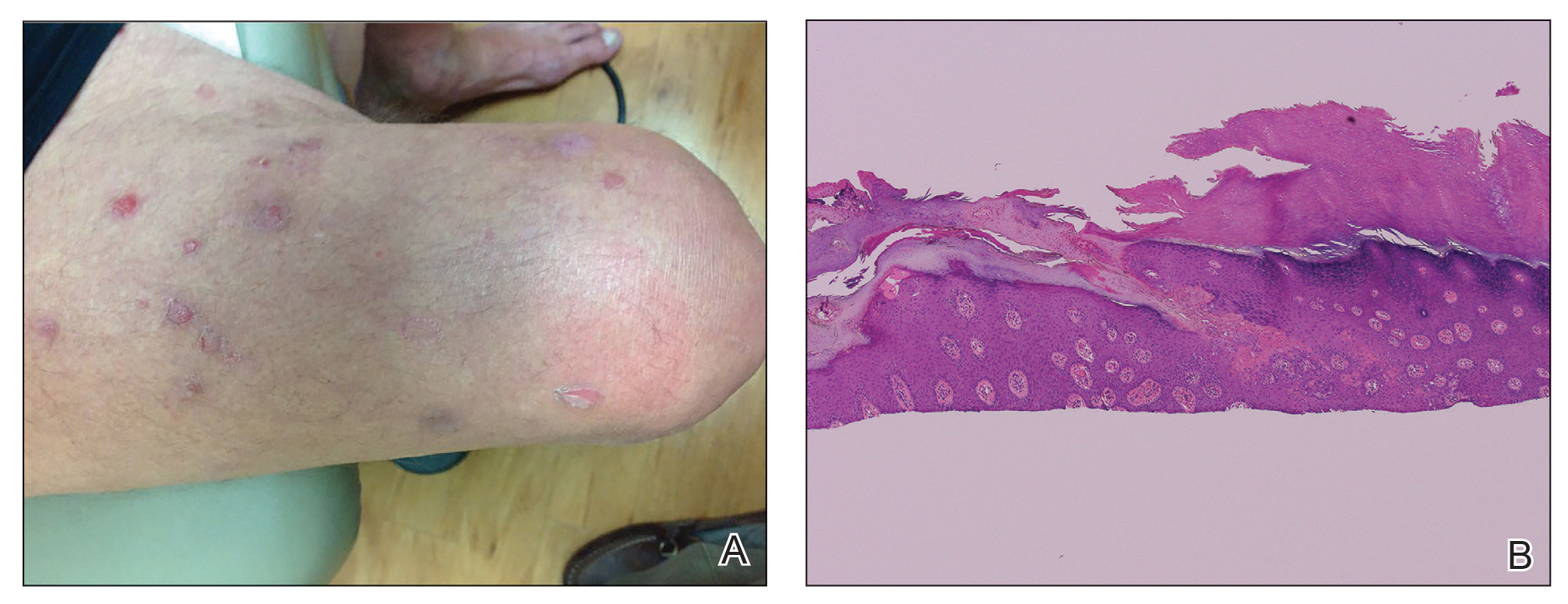
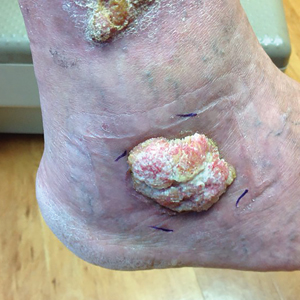
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
Practice Points
- The use of biologics, particularly tumor necrosis factor α blockers, rarely are reported to induce skin cancer.
- Squamous cell carcinoma in the setting of biologic treatment would warrant a change of systemic medication.