User login
Pharmacologic management of autism spectrum disorder: A review of 7 studies
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.
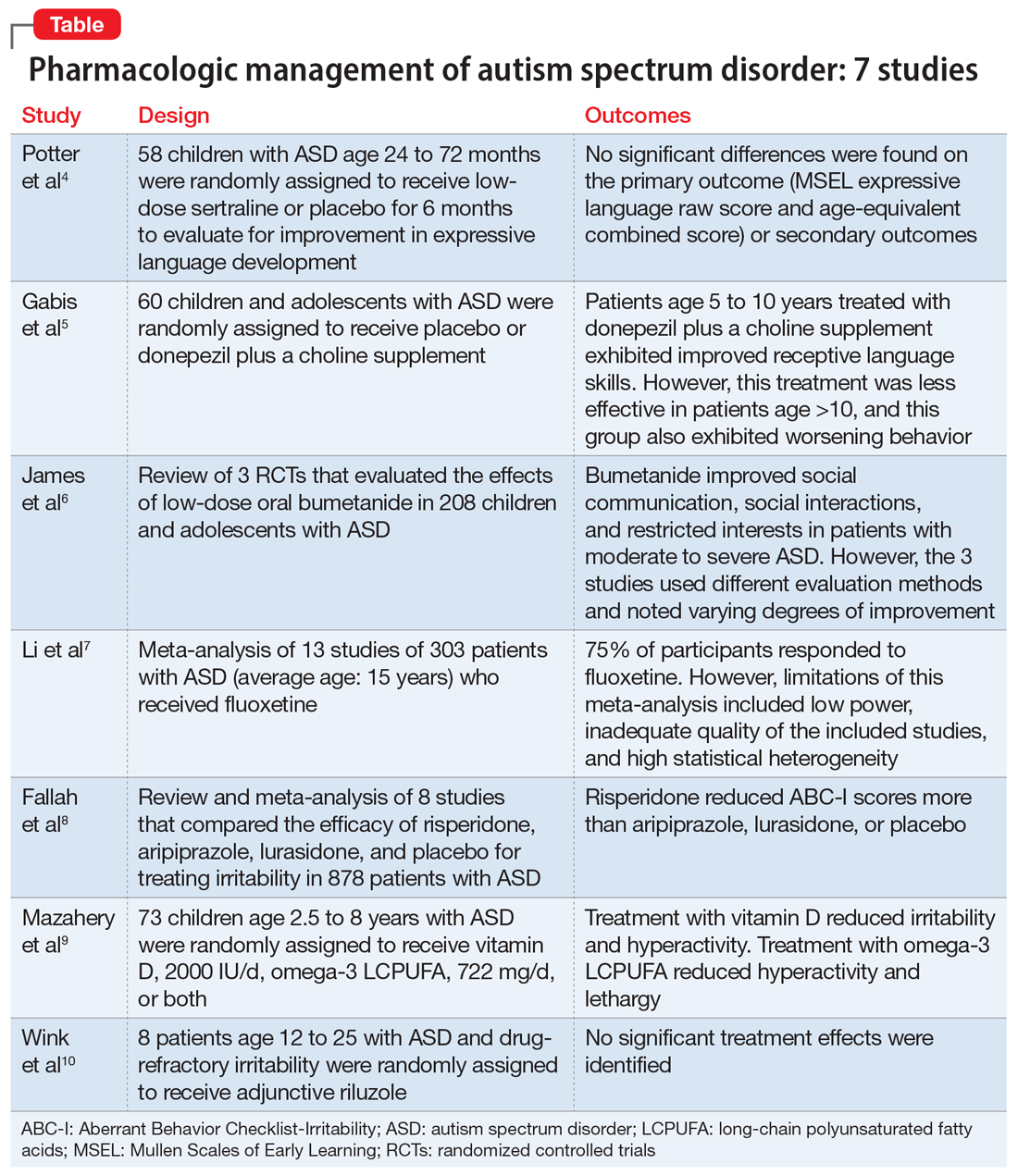
1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
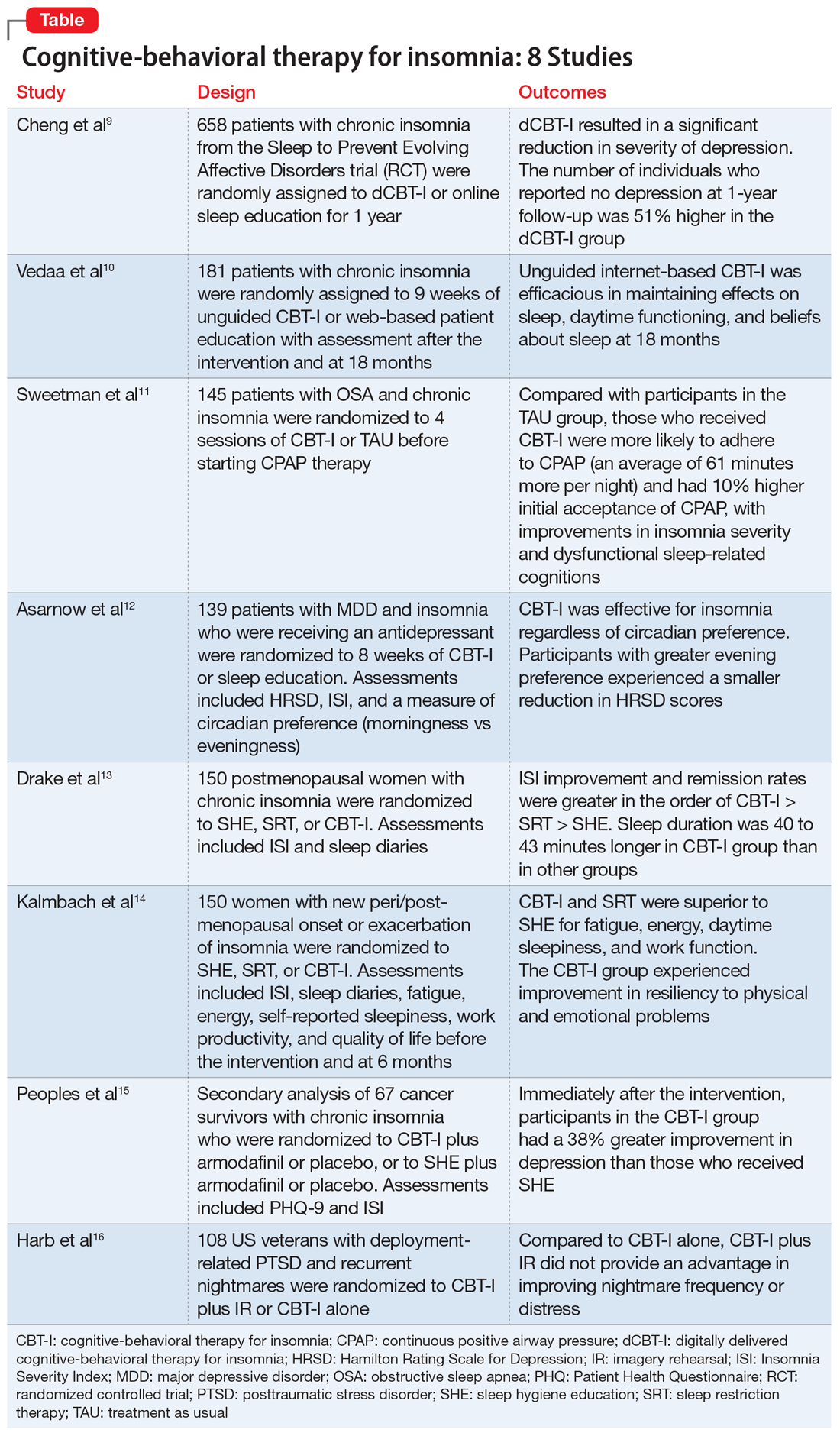
The psychiatric consequences of COVID-19: 8 Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
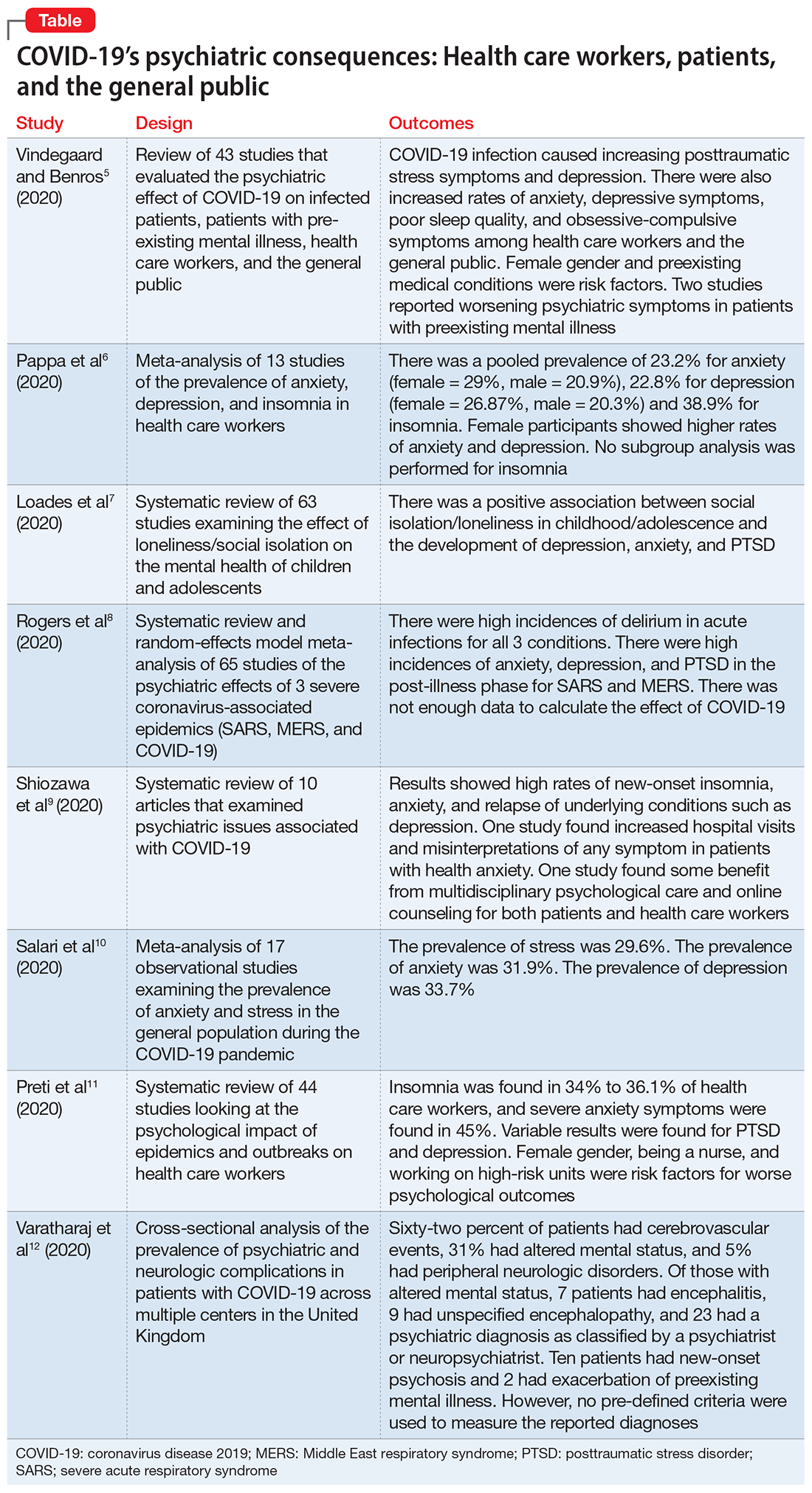
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Cognitive-behavioral therapy for insomnia: A review of 8 studies
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
Cannabidiol for psychosis: A review of 4 studies
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in
1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Top research findings of 2018-2019 for clinical practice
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.