User login
Top research findings of 2018-2019 for clinical practice
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
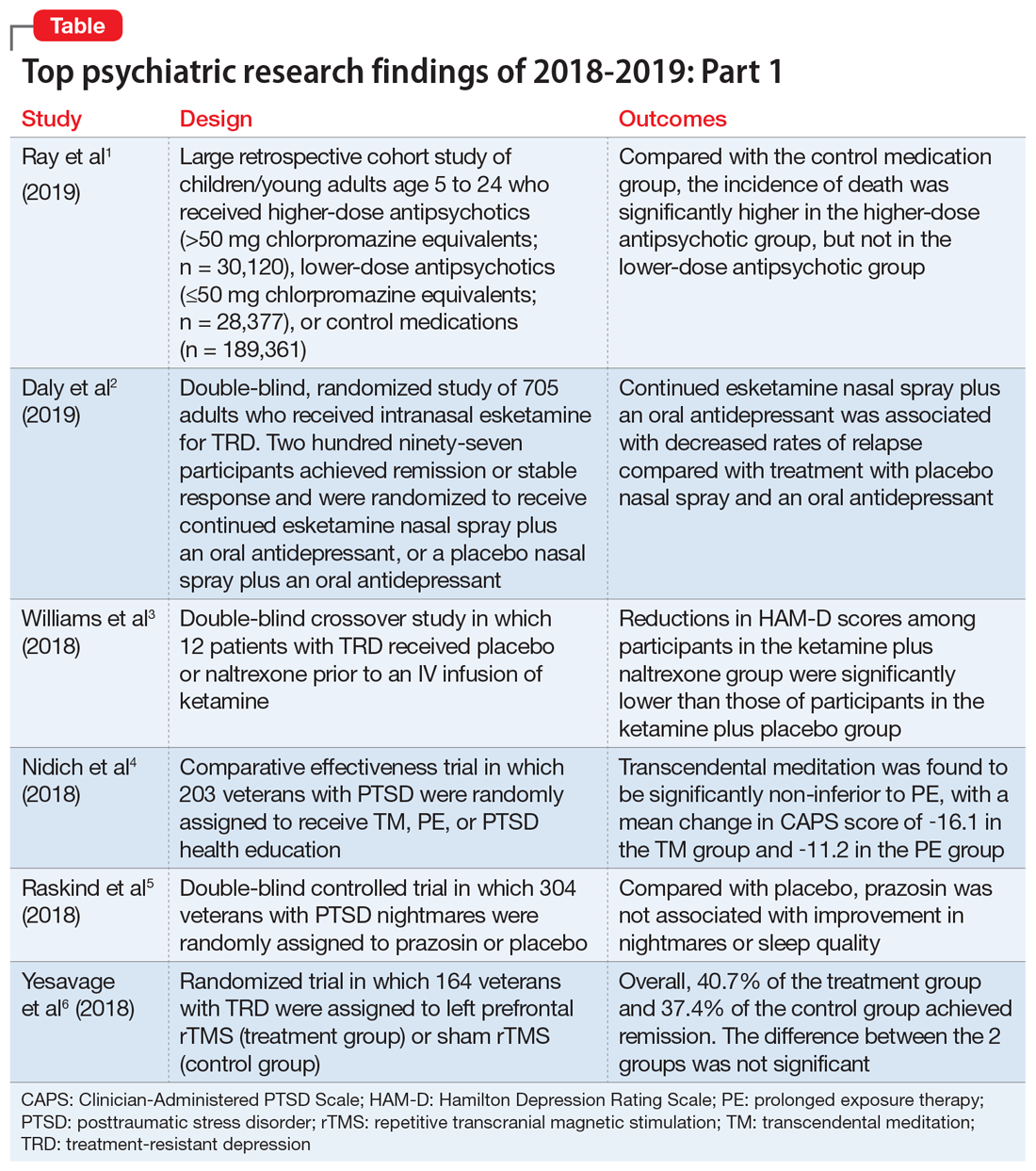
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
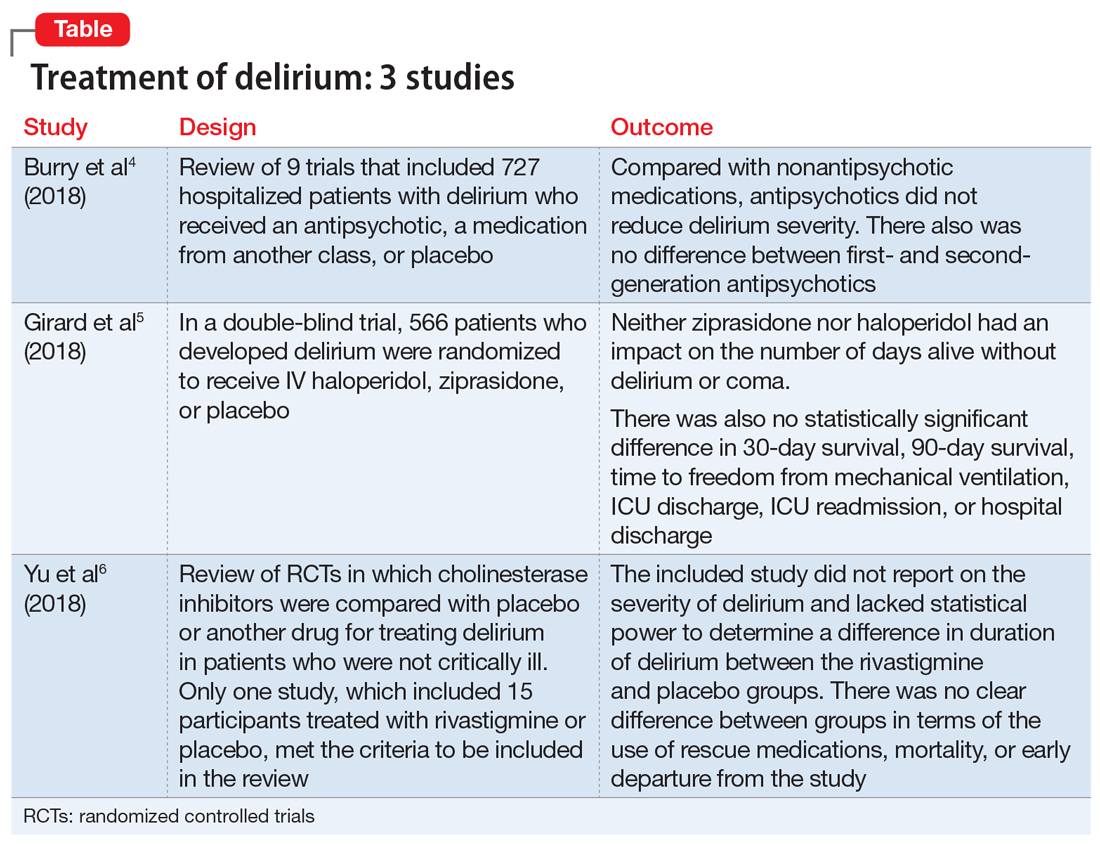
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.