User login
Psychotherapy for psychiatric disorders: A review of 4 studies
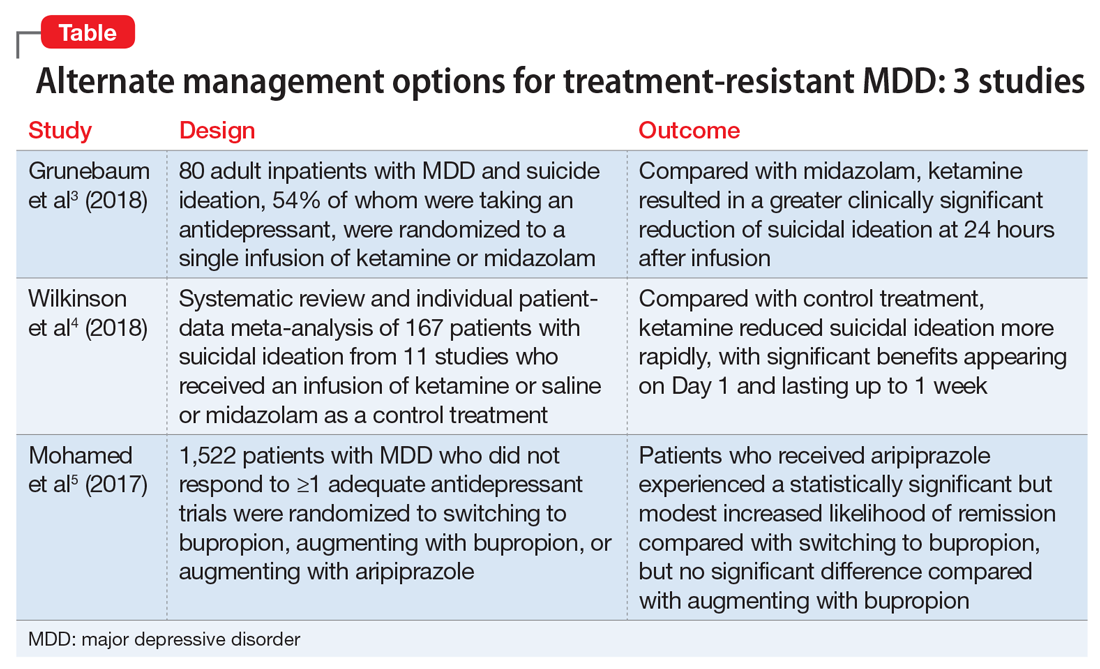
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
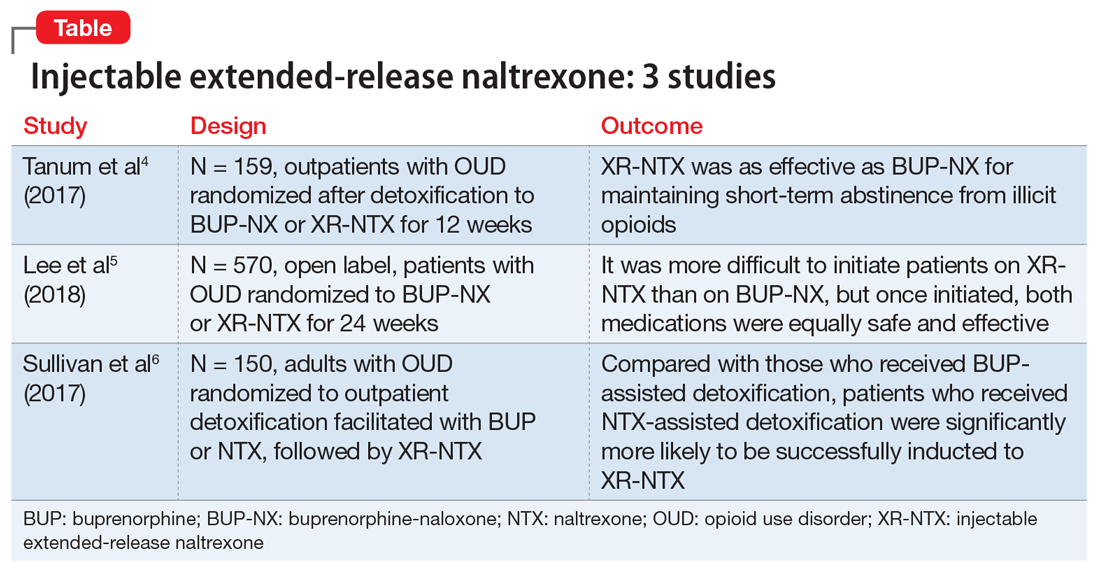
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
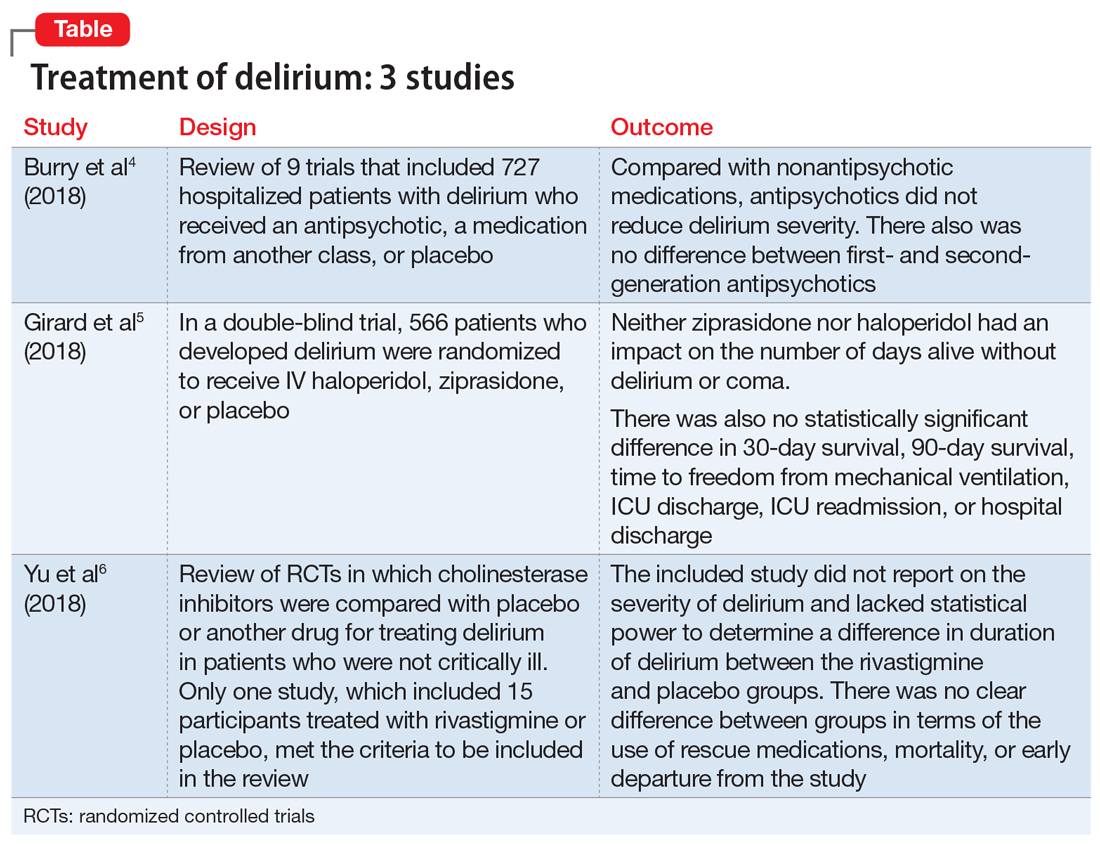
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Gut microbiota and its implications for psychiatry: A review of 3 studies
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
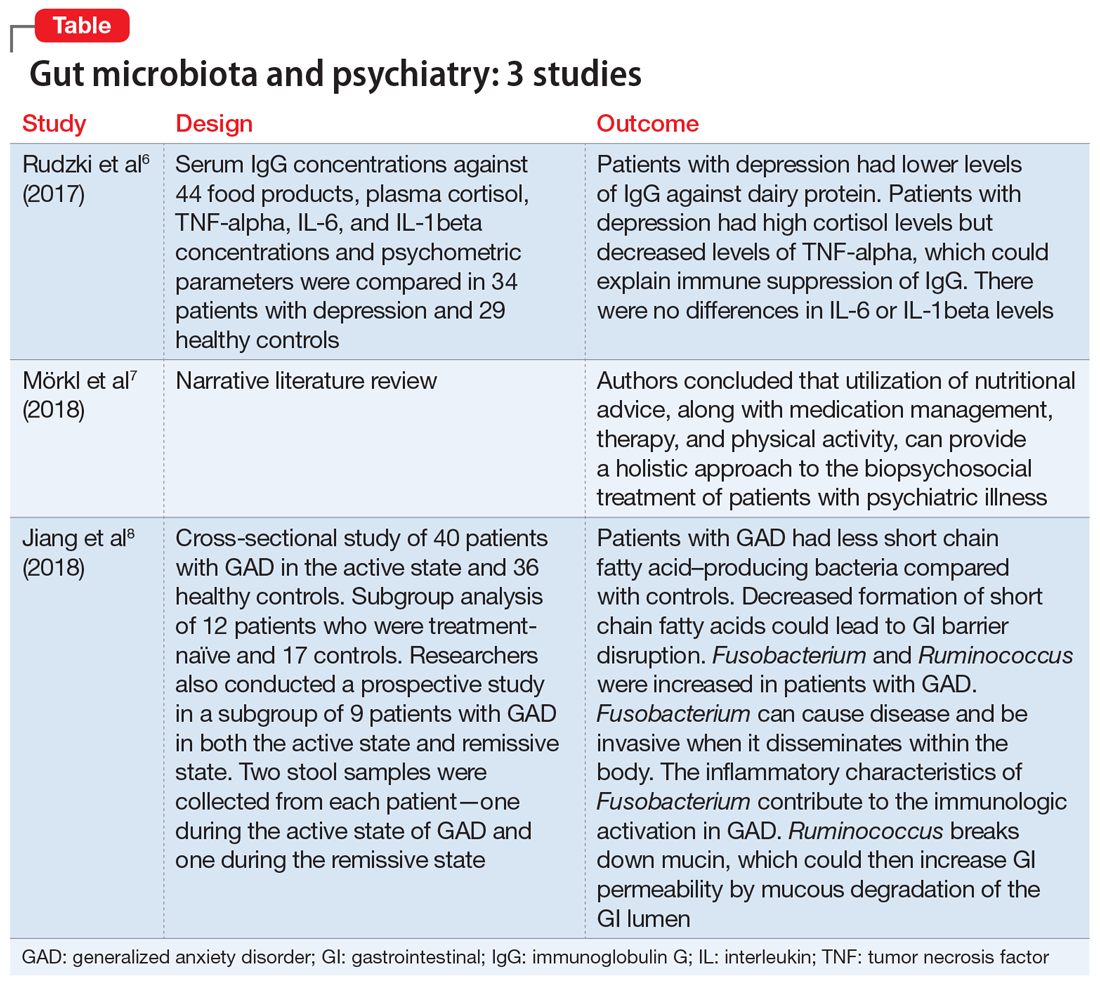
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
Management of treatment-resistant depression: A review of 3 studies
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Injectable extended-release naltrexone for opioid dependence: 3 studies
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Telepsychiatry: Overcoming barriers to implementation
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
CAM for your depressed patient: 6 recommended options
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-