User login
Breast cancer screening in women receiving antipsychotics
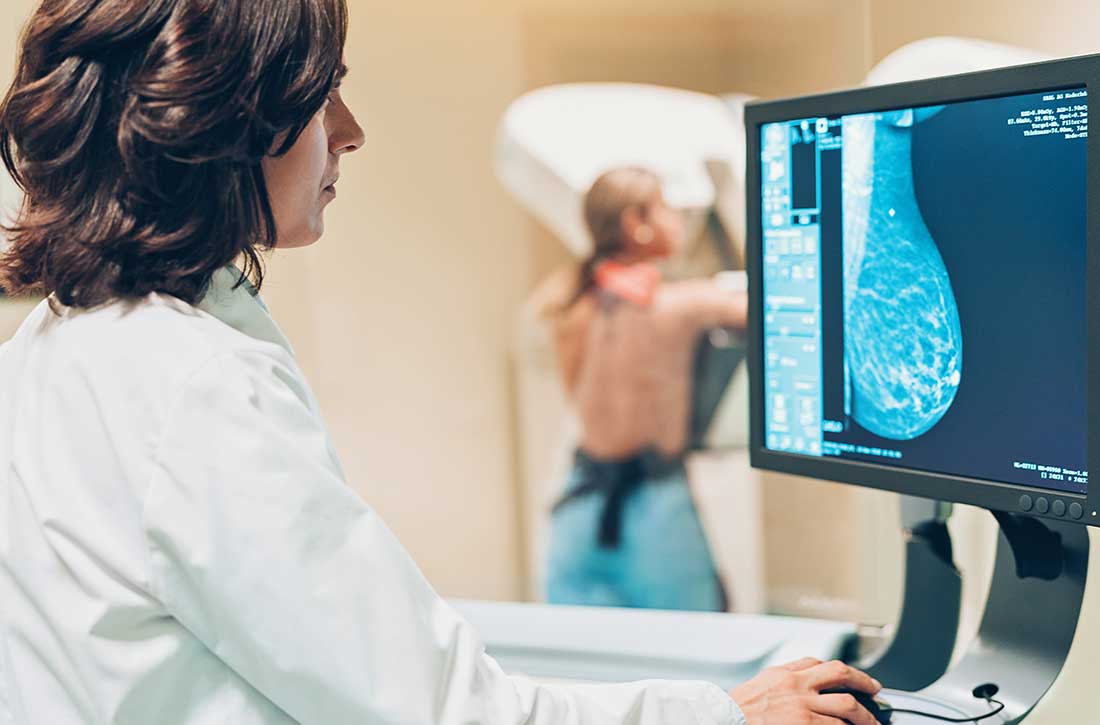
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Can mood stabilizers reduce chronic pain in patients with bipolar disorder?
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
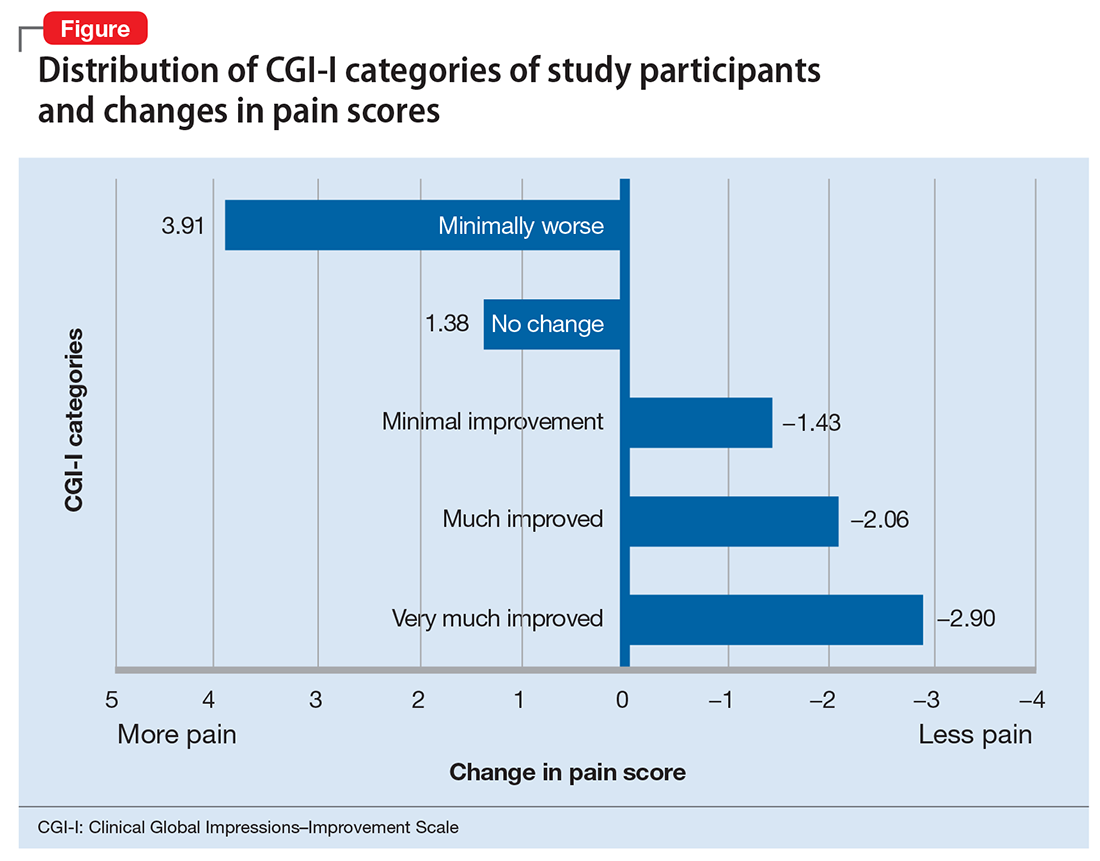
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).
Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).
Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).
Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
Breast cancer
Manic and nonadherent, with a diagnosis of breast cancer
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
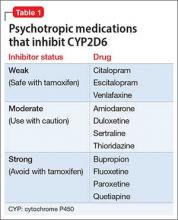
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
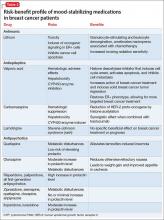
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.