User login
Transient Symmetric Blanching Macules on a Background of Reticulate Erythema
The Diagnosis: BASCULE Syndrome
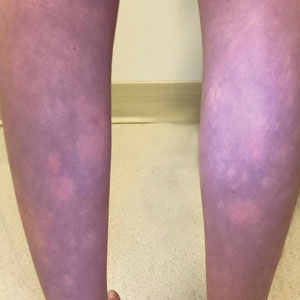
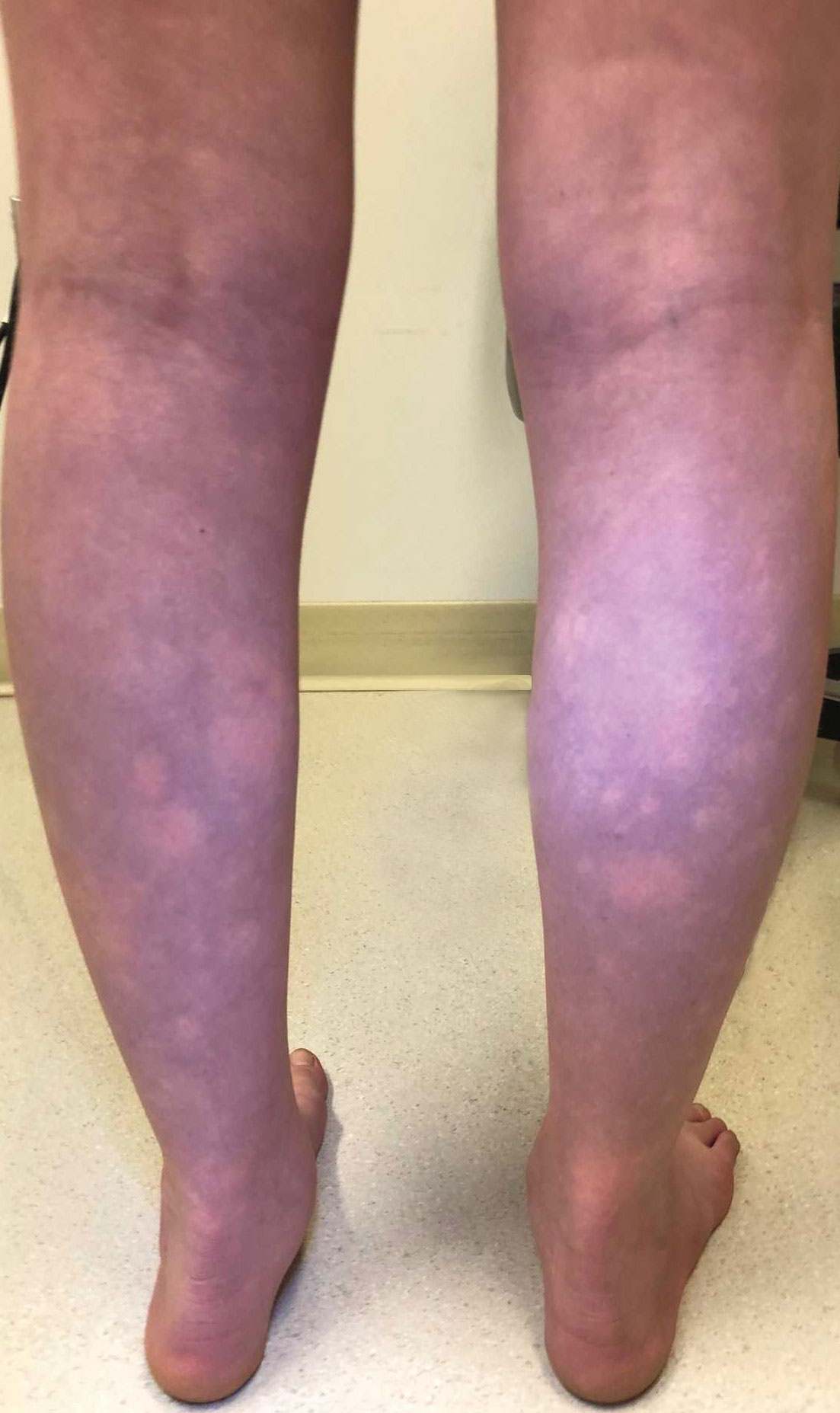
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
An 11-year-old girl was referred to the dermatology clinic for evaluation of a rash on the legs and feet of 1 year’s duration. The rash appeared every time she was standing for longer than 10 to 15 minutes and resolved when sitting or laying down. After the initial onset, the rash did not spread to other body areas but became more prominent in appearance. The patient endorsed intense pruritus associated with the rash. A review of systems was negative for fever, headaches, history of blood clots, and joint pain. She did not have any known medical conditions or take any medications. The patient’s mother reported that the patient experienced episodes of leg numbness while sitting in vehicles from 6 to 10 years of age. There was no family history of rheumatologic, hematologic, or cardiac conditions. The patient’s mother had experienced 2 miscarriages but denied any other obstetric complications. The patient had 1 sibling who was unaffected. Physical examination revealed reticulate erythema on the calves with scattered regions of blanching and evanescent pink macules as well as dermatographism.
One month prior to presenting to dermatology, the patient was evaluated by rheumatology, endocrinology, and hematology. Laboratory workup completed at age 3 years included antinuclear antibody, anticardiolipin antibody, and antithrombin III activity; factor V Leiden; cryoglobulins; quantitation (human chorionic gonadotropin); proteins S and C activity; antineutrophil cytoplasmic antibody screen; thyroid studies; prothrombin time; and partial thromboplastin time. All laboratory results were within reference range.
Woody Erythematous Induration on the Posterior Neck
The Diagnosis: Scleredema Diabeticorum
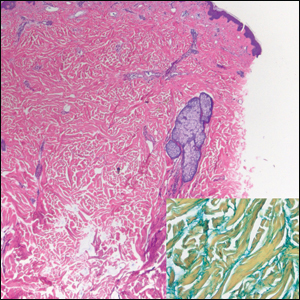
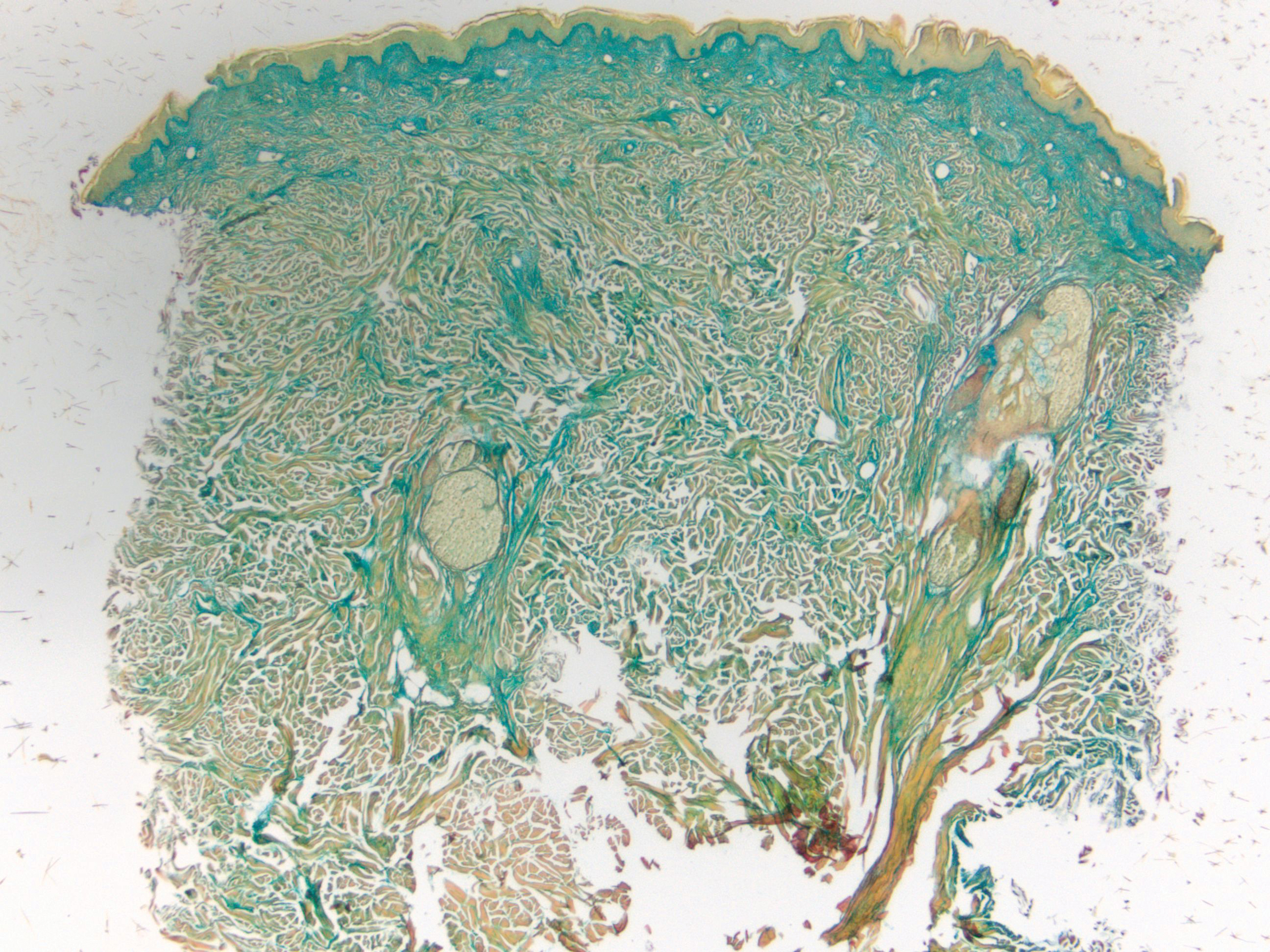
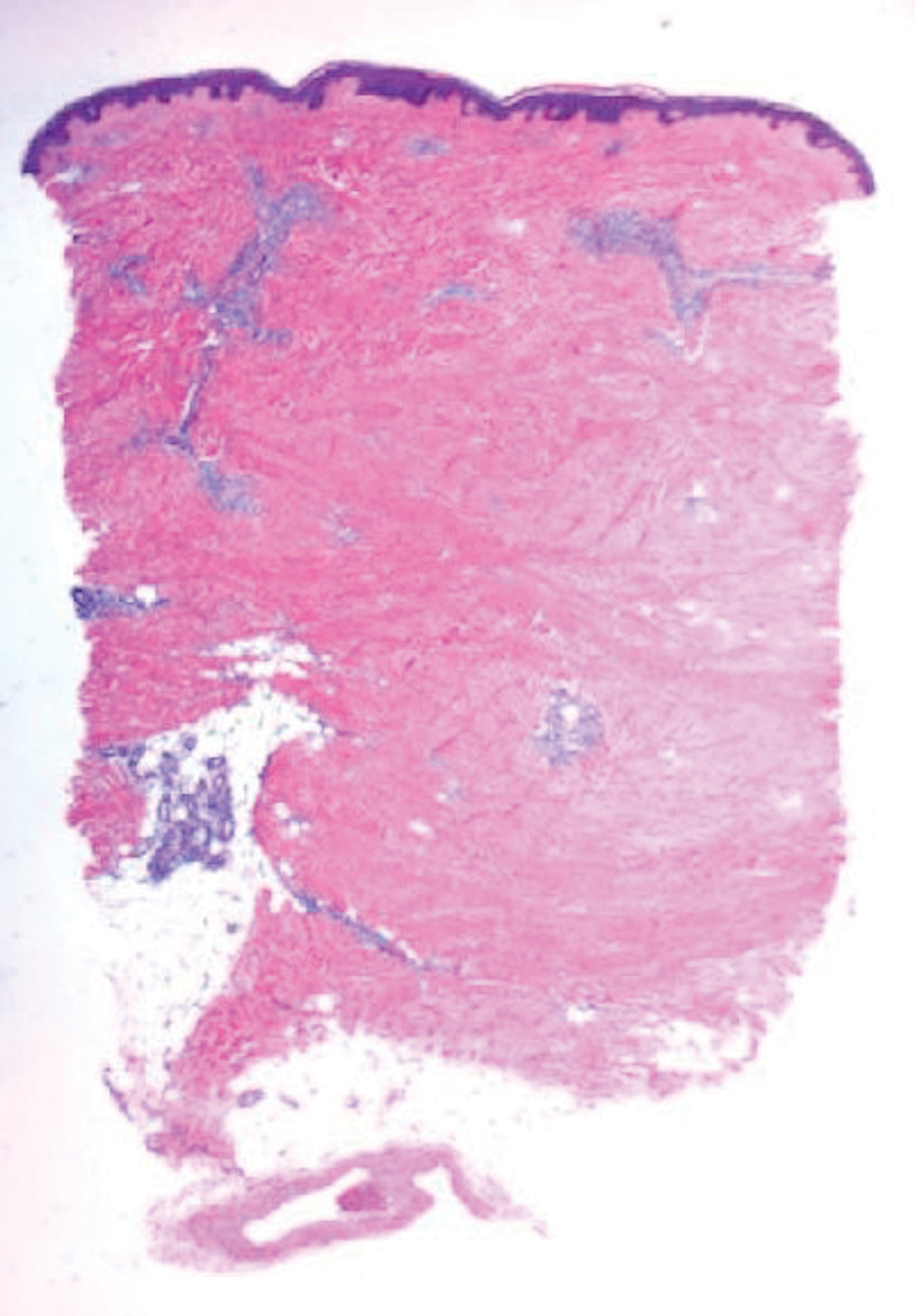
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1
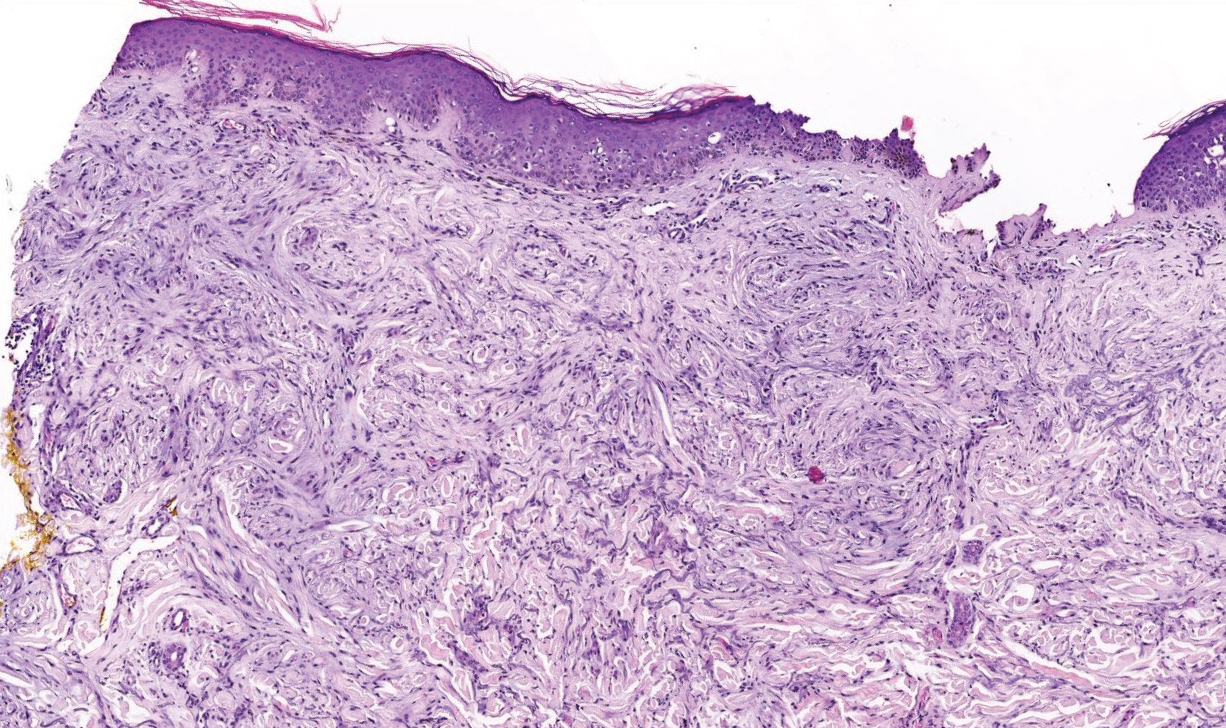
Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8

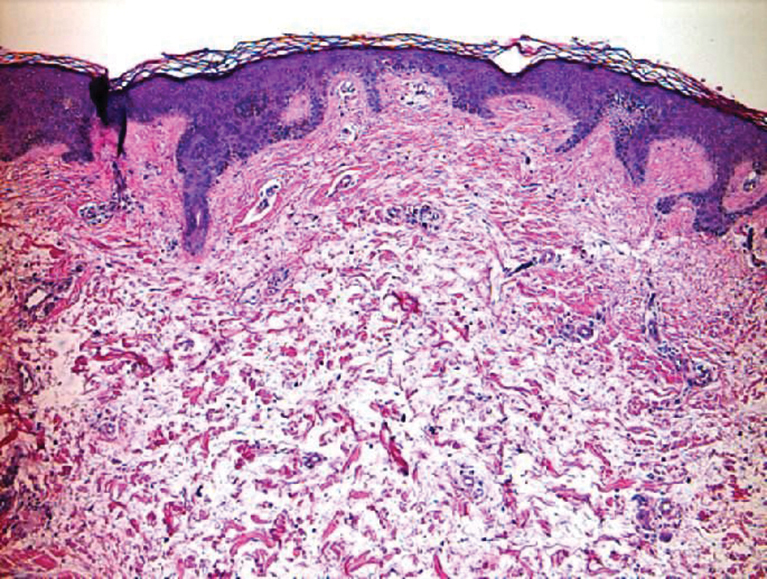
Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10
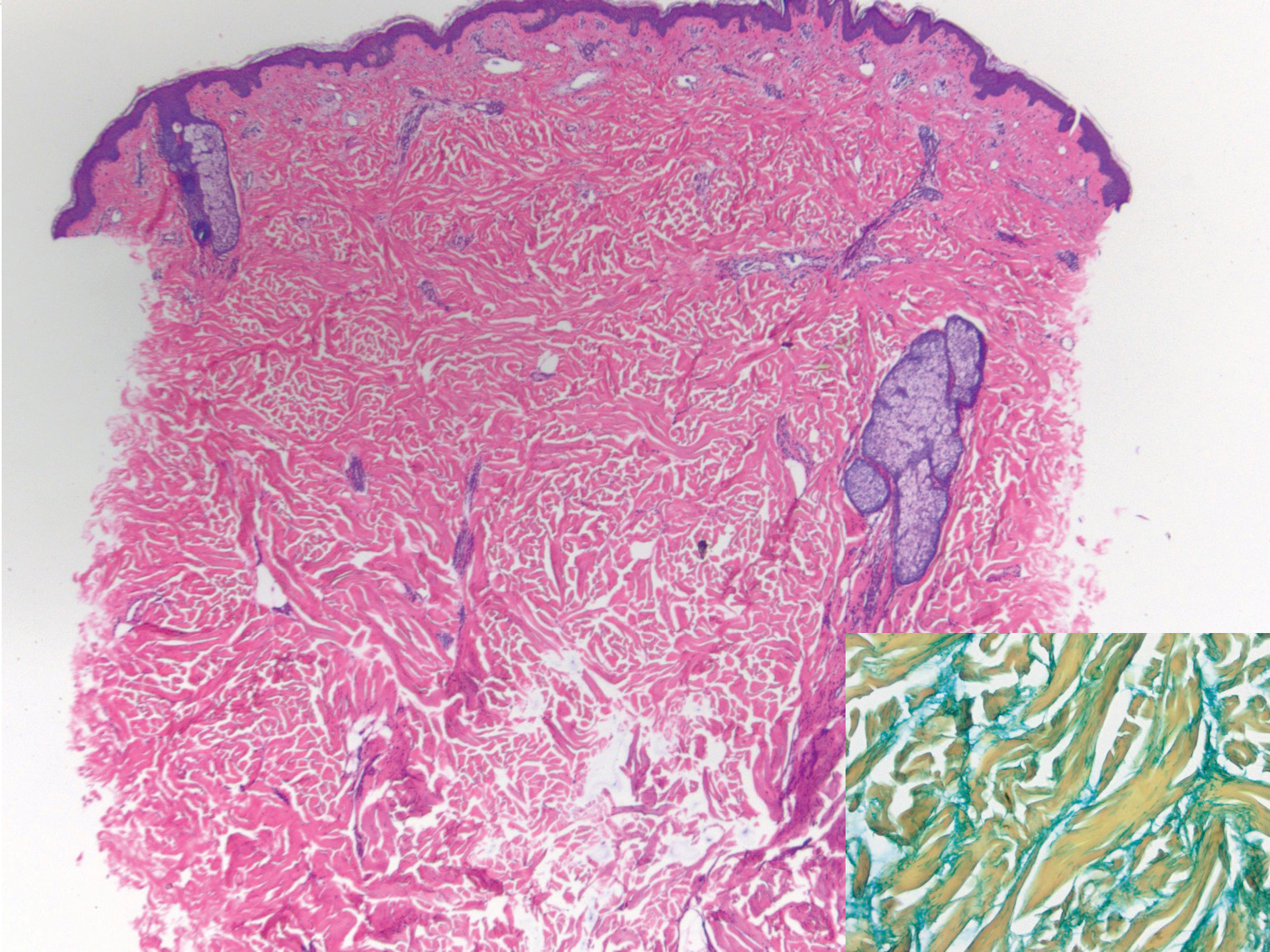
Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11
- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
The Diagnosis: Scleredema Diabeticorum
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1

Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8


Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10

Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11

The Diagnosis: Scleredema Diabeticorum
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1

Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8


Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10

Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11

- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
A 39-year-old white woman with a medical history of type 1 diabetes mellitus and rheumatoid arthritis presented to the dermatology clinic with pain and thickened skin on the posterior neck of 4 weeks’ duration. The patient noted stiffness in the neck and shoulders but denied any pain, pruritus, fever, chills, night sweats, fatigue, cough, dyspnea, dysphagia, weight loss, or change in appetite. Physical examination revealed a woody indurated plaque with slight erythema that was present diffusely on the posterior neck and upper back. The patient reported that a recent complete blood cell count and complete metabolic panel performed by her primary care physician were within reference range. Hemoglobin A1C was 8.6% of total hemoglobin (reference range, 4%–7%). A punch biopsy was performed.