User login
Extracorporeal membrane oxygenation in adults: A practical guide for internists
Extracorporeal membrance oxygenation (ECMO) provides temporary cardiorespiratory support for patients with severe respiratory or cardiac failure refractory to conventional therapy.1 It can be configured to provide oxygen, remove carbon dioxide, support perfusion, or all of the above. It may provide a bridge to recovery in patients with acute cardiopulmonary failure or to heart or lung transplant.
Developed in the 1970s, ECMO has proven effective and is widely used in children with respiratory and cardiopulmonary failure.2 However, it remained little used in adults, as early randomized trials showed higher rates of complications in adults who received it and no survival advantage.3,4 Proponents of using it in adult patients believe that these poor outcomes were at least partially due to limited training, intensive anticoagulation, and excessive volume and pressure during mechanical ventilation. Although ECMO technology has improved substantially in the last decade and survival rates have improved (www.elso.org), evidence to support its routine use in adults remains limited.
Nevertheless, about 14,000 adult patients received ECMO between 1990 and 2014, with a rate of survival to discharge of 57% for those in respiratory failure and 41% for those in cardiac failure.5 Its use increased 433% in the United States from 2006 to 2011.6
A national survey of critical care physicians and trainees in the United States found they had limited knowledge about ECMO technology and wanted to include specific educational objectives about it in their training.7
This article summarizes the principles of ECMO, including practical aspects such as patient selection, monitoring, and complications.
LIMITED EVIDENCE OF BENEFIT FROM CONTROLLED TRIALS
There is limited evidence from randomized controlled trials that ECMO is beneficial in adults.
In acute respiratory failure, the first randomized trial of ECMO in adults was conducted in 1979 in multiple medical centers.3 The survival rate was no higher with ECMO than with mechanical ventilation alone, and complication rates were very high.
Similarly, Morris et al4 performed a single-center trial comparing pressure-controlled inverse-ratio ventilation and extracorporeal carbon dioxide removal in patients with acute respiratory distress syndrome, which showed no survival benefit.
After these two early trials, ECMO was largely abandoned, and not until 2009 did a multicenter randomized trial in acute respiratory distress syndrome8 rejuvenate interest in its use. Although the trial did not conclusively prove that ECMO was more effective than conventional mechanical ventilation, the findings supported early referral to tertiary care centers with ECMO expertise, and the survival rate was substantially higher than in previous studies. A concise summary of randomized trials and retrospective studies utilizing ECMO in respiratory failure is shown in Table 1.8–14
During the global pandemic of influenza H1N1 in 2009–2010, several centers reported survival benefits from ECMO in patients with severe acute respiratory distress syndrome secondary to influenza.9–12,15–19 Two retrospective case-control studies reported lower mortality rates when H1N1 patients were transferred to ECMO centers10 and among younger patients with H1N1 who received ECMO.12
Ongoing trials (ClinicalTrials.gov identifier NCT01470703) may provide definitive evidence for the effectiveness of ECMO as a rescue therapy in acute respiratory distress syndrome.
In cardiogenic shock, single-center retrospective and observational studies have reported better outcomes for patients who received ECMO for cardiogenic shock secondary to myocardial infarction, pulmonary embolism, sepsis-related cardiomyopathy, and even extracorporeal cardiopulmonary resuscitation.20
WHAT IS ECMO?
In ECMO, venous blood is shunted through a machine to add oxygen, remove carbon dioxide, and regulate temperature (Figure 1). The components of an ECMO circuit are as follows:
- Blood pump
- Membrane oxygenator
- Gas mixer
- Cannulas
- Heater/cooler
- Console.
TWO BASIC CONFIGURATIONS
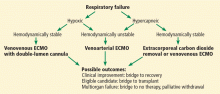
Two basic ECMO configurations are used in adults: venoarterial and venovenous,21 although combinations of the two—hybrid configurations—are sometimes used (Figure 2).
Venoarterial ECMO
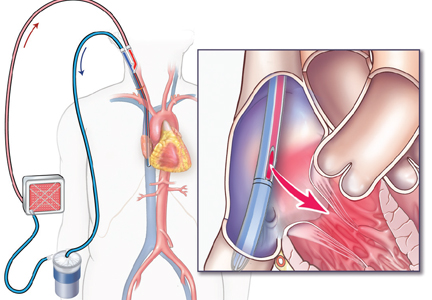
Venoarterial ECMO provides complete or partial support to the heart and lungs and is the configuration of choice in patients with isolated cardiac failure that is refractory to other treatments. It takes deoxygenated blood from the venous system and returns oxygenated blood to the arterial circulation.
In the central venoarterial configuration, the intake cannula is most often surgically placed in the right atrium and the return cannula is placed in the proximal ascending aorta.
In the peripheral femoral configuration, the drainage cannula is placed in the femoral vein and advanced to the right atrium, and the return cannula is placed in either the ipsilateral or contralateral femoral artery. However, this configuration provides the patient with retrograde flow (against the native cardiac output), and oxygen delivery to the upper body may be impeded.
Axillary cannulation, in which the return cannula is placed directly into the axillary artery to provide antegrade flow, has been used recently in patients with pulmonary hypertension or right ventricular failure.22
Venovenous ECMO
Venovenous ECMO provides complete or partial support to the lungs and is the configuration of choice in isolated respiratory failure when cardiac function is preserved. It takes deoxygenated blood from the central venous system—either the femoral vein or internal jugular vein—and returns oxygenated blood to the venous circulation directed into the right atrium. It can be delivered by different cannula configurations based on the patient’s size and clinical requirements.
In the past, the most commonly used configuration was the femoral-atrial, in which the drainage cannula was placed in the femoral vein with the tip advanced to the level of the diaphragm in the inferior vena cava, and the return cannula was placed in the right internal jugular vein with its tip at the junction of the superior vena cava and right atrium. In this configuration, some of the oxygenated blood delivered by the superior vena cava cannula reaches the inferior vena cava cannula, creating a “shunt,” also known as “recirculation.”
Currently, a double-lumen cannula is preferred. This type of cannula is placed in the right internal jugular vein with the tip advanced to the inferior vena cava so that blood is drained through one lumen from both the inferior and superior vena cavas and returned via the other lumen with the jet directed over the tricuspid valve. Advantages of this system are that as it delivers more oxygen to the pulmonary arteries it reduces recirculation, it requires only a single cannula to be inserted, and it facilitates ambulation and rehabilitation in patients requiring long-term ECMO.
A newer double-lumen cannula designed to drain venous blood from the right atrium and reinfuse it directly into the pulmonary artery may provide an alternative for patients with right ventricular failure.
Extracorporeal removal of carbon dioxide
ECMO can remove carbon dioxide in patients with hypercapneic respiratory failure. Early technology used a variation of venovenous ECMO with very low blood flow rates through the pump, which allowed use of smaller cannulas while efficiently removing carbon dioxide.23
Since then, a pumpless extracorporeal lung-assist device has been developed that uses an arteriovenous configuration with two smaller cannulas inserted into the femoral artery and vein (Novalung, Germany).24 Lacking a pump, it avoids the complications associated with pumps such as hemolysis and clotting. It effectively removes carbon dioxide and helps reduce the frequency and intensity of mechanical ventilation. Since the flow is driven by the patient’s arteriovenous pressure gradient, good cardiac output is a prerequisite for its use.
A portable low-blood-flow machine that uses a very small (ie, 15-F) catheter in the venovenous configuration is under investigation (Hemolung RAS, Alung Technologies).
WHO CAN BENEFIT FROM ECMO?
Although evidence to support the routine use of ECMO is limited, tools and guidelines have been developed to help clinicians decide if a patient might benefit from it. Indications for and contraindications to ECMO are shown in Table 2.
The Extracorporeal Life Support Organization recommends considering ECMO if the predicted risk of death is greater than 50% without it, and says ECMO is indicated if the predicted risk exceeds 80%. A scoring system has been developed to help predict the risk of death in patients on ECMO.14 This system has been validated using a historical cohort of patients, and current studies are ongoing for prospective validation.
Many centers are now using ECMO as a salvage therapy in patients with severe respiratory failure when conventional mechanical ventilation and adjunctive therapies such as neuromuscular blockade, inhaled nitric oxide, steroids, prone positioning, and high-frequency oscillation therapy fail to improve gas exchange.25,26
ECMO is also indicated in hypercapneic respiratory failure secondary to status asthmaticus and exacerbation of chronic obstructive pulmonary disease, permissive hypercapnea with a Paco2 greater than 80 mm Hg, or inability to achieve safe inflation pressures with plateau pressures of 30 cm H2O or higher, refractory to conventional therapy.27
Sometimes, delay in referral leads to irreversible ventilator-induced lung injury due to intense mechanical ventilation, thus limiting the utility of ECMO.8 Early referral should be considered if the patient does not improve after a few days on optimal ventilator settings. In centers where this technology is not available, referral to the nearest ECMO center should be considered. A list of certified ECMO centers is available at www.elso.org/Members/CenterDirectory.aspx.
Contraindications to ECMO
Advanced age, comorbid conditions such as malignancy, nonpulmonary organ dysfunction (including complications of critical illness), and immunodeficiency or pharmacologic immune suppression have been associated with poor outcomes in ECMO patients.28 Severe aortic incompetence and aortic dissection are contraindications, since ventricular end-diastolic pressure can be increased with resultant ventricular distention, compromised myocardial oxygenation, and worsening of left heart failure.
ECMO is increasingly being used in situations in which it was previously considered contraindicated. Pregnant and postpartum patients with cardiorespiratory failure were previously not considered for ECMO because of a possible increased risk of coagulopathy and complications. However, a recent review showed that the outcomes of ECMO in pregnancy and postpartum were similar to those in nonpregnant patients, and the risk of catastrophic bleeding was minor.29
Similarly, ECMO is also being used increasingly in posttrauma patients and patients with other bleeding risks.30
Morbid obesity was once considered a contraindication because of difficulty in cannulation, but with newer types of cannulas, even patients with a body mass index greater than 60 kg/m2 are receiving ECMO.31
HOW DO YOU DO IT?
Figures 3 and 4 depict clinical decision-making in starting and weaning from ECMO in respiratory failure and cardiogenic shock, respectively.
Management of patients on ECMO
Appropriate patient selection and initiation of ECMO are only the beginning of a tough journey. Successful management requires minimizing lung injury from mechanical ventilation, careful monitoring of anticoagulation, and instituting adequate physical therapy, including ambulation when possible (Table 3).
Initial ECMO settings and monitoring
The cannulas for venovenous ECMO are frequently inserted under fluoroscopic or transesophageal echocardiographic guidance, whereas venoarterial ECMO cannulation does not require imaging and can be performed at the bedside in the intensive care unit or operating room.
The initial ECMO settings are titrated according to the patient’s hemodynamic and respiratory needs. There are three main variables: blood flow, fraction of oxygen in the sweep gas, and sweep gas flow rate. These are adjusted to achieve desirable levels of oxygen and carbon dioxide in the blood.
Blood flow is determined by the revolutions per minute of the pump, preload, and afterload of the circuit. Common patient conditions that may reduce flow are systemic hypertension, hypovolemia, cardiac tamponade, and tension pneumothorax, depending on the modality. In addition, mechanical factors such as clots in the oxygenator or kinks in the circuit can increase resistance and reduce flow. Resistance to flow is directly proportional to cannula lengths and inversely proportional to cannula radius to the fourth power. The greater the flow, the greater the oxygen delivery.
Fraction of oxygen in the sweep gas. The oxygenator has a gas blender that mixes air and oxygen and allows for a range of oxygen concentrations. Increases in fraction of oxygen increase the partial pressure of oxygen in the blood.
Sweep gas flow rate. Venous blood in the extracorporeal circuit is exposed to fresh gas (or sweep gas) that oxygenates the blood and removes carbon dioxide by diffusion. Increasing the sweep gas flow rate results in greater carbon dioxide elimination from the blood.
Laboratory monitoring. During ECMO, the following values are monitored frequently:
- Arterial blood gases
- Blood gases in the ECMO circuit before and after going through the oxygenator— to monitor the efficacy of the oxygenator membrane
- Lactic acid—to monitor for tissue hypoxia
- Plasma free hemoglobin (a marker of hemolysis)—to monitor for hemolysis.
Mechanical ventilation on ECMO
Low tidal volume ventilation greatly reduces the risk of death in patients on ECMO by reducing ventilator-induced lung injury. Proponents of ECMO believe that ECMO provides “lung rest,” and thus it is imperative that lung-protective ventilation strategies be followed in patients on ECMO.8 In most cases, after ECMO is started, low tidal volume ventilation (6 mL/kg) is possible and should be used—or even very low tidal volume ventilation (3–6 mL/kg).32,33 Many cases have also been described in which patients have been safely extubated while on ECMO to prevent ventilator-induced lung injury.34,35
If hypoxemia persists
Despite full support with venovenous ECMO, some patients remain hypoxemic due to inadequate blood flow to match metabolic demands, eg, patients with morbid obesity or severe sepsis and fever. The physician should ensure there is no recirculation, maximize blood flow, optimize the hematocrit to increase oxygen delivery, and consider ways to decrease oxygen consumption, including sedation, paralysis, and hypothermia.
Recirculation can be calculated by measuring the oxygen saturation of the blood in the ECMO machine before and after it goes through the oxygenator, and also in the central venous blood. Recirculation has been reduced by using double-lumen cannulas but can also be reduced by manipulation of the reinfusion cannula or increasing the distance between drainage and reinfusion ports in other configurations of venovenous ECMO.
Expert opinion suggests that oxygen saturation of 86% or more and Pao2 of 55 mm Hg or more in patients on venovenous ECMO are sufficient to prevent hypoxia-related end-organ injury.36 Venoarterial ECMO should be considered in patients on venovenous ECMO with refractory hypoxemia with the above measures.
Harlequin syndrome is characterized by upper body hypoxia resulting in cerebral hypoxemia due to poorly oxygenated blood in the coronary and cerebral circulations, especially in patients on peripheral venoarterial ECMO. It can be detected by sampling the blood in the arm (where the oxygen isn’t going) instead of the leg (where the oxygen is going), and it can be corrected by adjusting the Fio2, using positive end-expiratory pressure, or both to increase oxygenation. If ventilator settings do not improve this syndrome, the arterial cannulation site can be switched from the femoral artery to the axillary or carotid artery.
Alternatively, a mixed-configuration venoarterial-venous ECMO can also be created, in which a portion of arterialized blood from the arterial outflow cannula is diverted via the right internal jugular artery to the right heart. This enriches the blood traveling through the pulmonary circulation and to the left ventricle to provide better oxygen delivery to the coronary and cerebral circulations.
Anticoagulation monitoring and transfusions
Anticoagulation is necessary to maintain a clot-free and functional circuit. Most clots develop in the oxygenator membrane, where they can prevent optimal gas exchange and, rarely, lead to embolization to the systemic circulation. However, reports have suggested that anticoagulation can be held for short periods on ECMO if necessary.
Unfractionated heparin is usually used for anticoagulation. Commonly used tests to monitor anticoagulation are the augmented partial thromboplastin time, activated clotting time, and anti-factor Xa levels. Lately, thromboelastography analysis is being used to comprehensively monitor various components of the coagulation cascade.37 Anticoagulation is usually tailored to whether there are clots in the circuit, coagulopathy, and bleeding while on ECMO.38
Traditionally, blood products were used liberally during ECMO to maintain a normal hematocrit and improve oxygen delivery, although recent data suggest that outcomes may be similar with conservative use of blood products.39,40
Fluid management on ECMO
ECMO patients are fluid-overloaded due to a profound inflammatory response, cardiac failure, or both. Studies have shown that conservative fluid management improve lung function and shortens the duration of mechanical ventilation and intensive care in patients with lung injury.41 Hence, the patient’s net fluid balance should be kept negative, provided renal and hemodynamic parameters remain stable.
There is a high incidence of acute kidney injury in ECMO patients, and fluid overload is one of the main indications for renal replacement therapy.42 Continuous renal replacement therapy can be provided either by an in-line hemofilter or by incorporating a standard continuous renal replacement therapy machine into the ECMO circuit. There are no studies comparing the efficacy of these techniques, but they allow for rapid improvement in fluid balance and electrolyte disturbances and are commonly used in ECMO patients.42,43
Physical rehabilitation and ambulation on ECMO
Physical rehabilitation in mechanically ventilated patients has been shown to reduce ventilator days and stay in the intensive care unit.44 With the use of internal jugular double-lumen cannulas for venovenous ECMO and improvement in durability of the ECMO circuit, several centers are implementing physical rehabilitation and ambulation for patients while on ECMO. Current data suggest that physical therapy is safe for patients receiving ECMO and may accelerate the weaning process and shorten length of stay in the hospital after ECMO.45,46 Aggressive rehabilitation is especially beneficial in patients awaiting lung transplant and may improve posttransplant recovery and outcomes.47
Weaning from ECMO
There are no standard guidelines for weaning from venovenous or venoarterial ECMO. Once the underlying condition for which ECMO was initiated has improved, weaning can begin by reducing the blood flow rate, the flow rate of the sweep gas, or both.
Weaning from venovenous ECMO should be started when there is improvement in lung compliance, tidal volumes, and oxygenation. Once the circuit flow rate is reduced to less than 3 L/minute, ventilator settings are adjusted to standard lung-protective settings. ECMO support is gradually decreased by reducing the flow rate of sweep gas to less than 2 L/minute. If tidal volumes, respiratory rate, and gas exchange remain adequate after approximately 2 to 4 hours on a low rate of sweep gas, the patient can be weaned off the venovenous ECMO circuit.
Weaning from venoarterial ECMO should be considered when there is myocardial recovery with improved pulse pressure and contractility on echocardiography. This is done by reducing flow rates in increments of 0.5 to 2 L/minute over 24 to 36 hours and monitoring mean arterial pressures, central venous pressure, and myocardial contractility. When acceptable, patients are mostly weaned in a surgical setting. Prolonged periods on a low rate of blood flow are avoided to prevent thrombus formation in the circuit.
COMPLICATIONS OF ECMO
ECMO use can be associated with a myriad of patient and mechanical complications.
Hemorrhage is the most common complication encountered in ECMO, occurring in approximately 43% of patients.29 It occurs most frequently from cannulation and surgical sites. Although rare, potentially life-threatening pulmonary hemorrhage (including bleeding at the tracheostomy site), intracranial hemorrhage, and gastrointestinal hemorrhage have also been reported.30
Infections, including new infection and worsening sepsis in patients with acute respiratory distress syndrome secondary to infection, are common in patients on ECMO.48
Renal failure secondary to acute tubular necrosis requiring hemodialysis has been reported to occur in 13% of patients on ECMO.30
Other complications of concern, especially in patients on venoarterial ECMO, are lower limb ischemia and thromboembolism associated with site of cannulation and direction of blood flow.49 Mechanical complications include inappropriate placement of the cannula leading to insufficient oxygenation, injury to vessel walls, and rarely myocardial wall rupture; thrombus formation within the circuit causing failure of the oxygenator and sometimes, pulmonary or systemic embolism; and air embolism from the circuit.36
NOT SUITED FOR ALL
Despite limited data to support its use, there has been a recent increase in utilization of ECMO to support critically ill adult patients with cardiopulmonary failure. ECMO support is not suited for all patients. Careful selection of patients should be done to optimize resource utilization and provide the best opportunity for recovery or transplant.
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012; 38:210–220.
- Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: history, development and current status. World J Crit Care Med 2013; 2:29–39.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Extracorporeal Life Support Organization. ECLS registry report. International Summary. January 2016. https://www.elso.org/Registry.aspx. Accessed March 17, 2016.
- Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015; 61:31–36.
- Sharma N, Wille K, Bellot S, Brodie D, Diaz-Guzman E. Role of extracorporeal membrane oxygenation in management of refractory ARDS in the intensive care unit: a national survey on perspectives of the adult critical care physicians and trainees. Chest 2014. http://journal.publications.chestnet.org/article.aspx?articleid=1913336. Accessed March 17, 2016.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302:1888–1895.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011; 37:1447–1457.
- Pham T, Combes A, Roze H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39; 532:1704–1713.
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189:1374–1382.
- Chan KK, Lee KL, Lam PK, Law KI, Joynt GM, Yan WW. Hong Kong's experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1). Hong Kong Med J 2010; 16:447–454.
- Freed DH, Henzler D, White CW, et al; Canadian Critical Care Trials Group. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth 2010; 57:240–247.
- Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 2011; 37:648–654.
- Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care 2011; 56:941–946.
- Kumar A, Zarychanski R, Pinto R, et al; Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879.
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014; 63:2769–2778.
- Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008; 17(suppl 4):S41–S47.
- Hysi I, Fabre O, Renaut C, Guesnier L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg 2014; 29:268–269.
- Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs 1979; 2:282–283.
- Liebold A, Philipp A, Kaiser M, Merk J, Schmid FX, Birnbaum DE. Pumpless extracorporeal lung assist using an arterio-venous shunt. Applications and limitations. Minerva Anestesiol 2002; 68:387–391.
- Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013; 59:202–210.
- Shekar K, Davies AR, Mullany DV, Tiruvoipati R, Fraser JF. To ventilate, oscillate, or cannulate? J Crit Care 2013; 28:655–662.
- Mikkelsen ME, Woo YJ, Sager JS, Fuchs BD, Christie JD. Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J 2009; 55:47–52.
- Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care 2013; 58:1038–1052.
- Sharma NS, Wille KM, Bellot SC, Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J 2015; 61:110–114.
- Ried M, Bein T, Philipp A, et al. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care 2013; 17:R110.
- Al-Soufi S, Buscher H, Nguyen ND, Rycus P, Nair P. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med 2013; 39:1995–2002.
- Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 2014; 11:956–961.
- Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014; 18:203.
- Bein T, Wittmann S, Philipp A, Nerlich M, Kuehnel T, Schlitt HJ. Successful extubation of an "unweanable" patient with severe ankylosing spondylitis (Bechterew's disease) using a pumpless extracorporeal lung assist. Intensive Care Med 2008; 34:2313–2314.
- Anton-Martin P, Thompson MT, Sheeran PD, Fischer AC, Taylor D, Thomas JA. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatr Crit Care Med 2014; 15:861–869.
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth 2010; 24:164–172.
- Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol 1995; 27:137–145.
- Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013; 59:63–68.
- Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015; 99:590–595.
- Voelker MT, Busch T, Bercker S, Fichtner F, Kaisers UX, Laudi S. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artif Organs 2015; 39:374–378.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J 2012; 58:407–414.
- Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2012; 7:1328–1336.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18:R38.
- Thiagarajan RR, Teele SA, Teele KP, Beke DM. Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med 2012; 5:47–52.
- Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013; 145:862–868.
- Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis 1999; 28:828–833.
- Lafç G, Budak AB, Yener AÜ, Cicek OF. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ 2014; 23:10–23.
Extracorporeal membrance oxygenation (ECMO) provides temporary cardiorespiratory support for patients with severe respiratory or cardiac failure refractory to conventional therapy.1 It can be configured to provide oxygen, remove carbon dioxide, support perfusion, or all of the above. It may provide a bridge to recovery in patients with acute cardiopulmonary failure or to heart or lung transplant.
Developed in the 1970s, ECMO has proven effective and is widely used in children with respiratory and cardiopulmonary failure.2 However, it remained little used in adults, as early randomized trials showed higher rates of complications in adults who received it and no survival advantage.3,4 Proponents of using it in adult patients believe that these poor outcomes were at least partially due to limited training, intensive anticoagulation, and excessive volume and pressure during mechanical ventilation. Although ECMO technology has improved substantially in the last decade and survival rates have improved (www.elso.org), evidence to support its routine use in adults remains limited.
Nevertheless, about 14,000 adult patients received ECMO between 1990 and 2014, with a rate of survival to discharge of 57% for those in respiratory failure and 41% for those in cardiac failure.5 Its use increased 433% in the United States from 2006 to 2011.6
A national survey of critical care physicians and trainees in the United States found they had limited knowledge about ECMO technology and wanted to include specific educational objectives about it in their training.7
This article summarizes the principles of ECMO, including practical aspects such as patient selection, monitoring, and complications.
LIMITED EVIDENCE OF BENEFIT FROM CONTROLLED TRIALS
There is limited evidence from randomized controlled trials that ECMO is beneficial in adults.
In acute respiratory failure, the first randomized trial of ECMO in adults was conducted in 1979 in multiple medical centers.3 The survival rate was no higher with ECMO than with mechanical ventilation alone, and complication rates were very high.
Similarly, Morris et al4 performed a single-center trial comparing pressure-controlled inverse-ratio ventilation and extracorporeal carbon dioxide removal in patients with acute respiratory distress syndrome, which showed no survival benefit.
After these two early trials, ECMO was largely abandoned, and not until 2009 did a multicenter randomized trial in acute respiratory distress syndrome8 rejuvenate interest in its use. Although the trial did not conclusively prove that ECMO was more effective than conventional mechanical ventilation, the findings supported early referral to tertiary care centers with ECMO expertise, and the survival rate was substantially higher than in previous studies. A concise summary of randomized trials and retrospective studies utilizing ECMO in respiratory failure is shown in Table 1.8–14
During the global pandemic of influenza H1N1 in 2009–2010, several centers reported survival benefits from ECMO in patients with severe acute respiratory distress syndrome secondary to influenza.9–12,15–19 Two retrospective case-control studies reported lower mortality rates when H1N1 patients were transferred to ECMO centers10 and among younger patients with H1N1 who received ECMO.12
Ongoing trials (ClinicalTrials.gov identifier NCT01470703) may provide definitive evidence for the effectiveness of ECMO as a rescue therapy in acute respiratory distress syndrome.
In cardiogenic shock, single-center retrospective and observational studies have reported better outcomes for patients who received ECMO for cardiogenic shock secondary to myocardial infarction, pulmonary embolism, sepsis-related cardiomyopathy, and even extracorporeal cardiopulmonary resuscitation.20
WHAT IS ECMO?
In ECMO, venous blood is shunted through a machine to add oxygen, remove carbon dioxide, and regulate temperature (Figure 1). The components of an ECMO circuit are as follows:
- Blood pump
- Membrane oxygenator
- Gas mixer
- Cannulas
- Heater/cooler
- Console.
TWO BASIC CONFIGURATIONS
Two basic ECMO configurations are used in adults: venoarterial and venovenous,21 although combinations of the two—hybrid configurations—are sometimes used (Figure 2).
Venoarterial ECMO
Venoarterial ECMO provides complete or partial support to the heart and lungs and is the configuration of choice in patients with isolated cardiac failure that is refractory to other treatments. It takes deoxygenated blood from the venous system and returns oxygenated blood to the arterial circulation.
In the central venoarterial configuration, the intake cannula is most often surgically placed in the right atrium and the return cannula is placed in the proximal ascending aorta.
In the peripheral femoral configuration, the drainage cannula is placed in the femoral vein and advanced to the right atrium, and the return cannula is placed in either the ipsilateral or contralateral femoral artery. However, this configuration provides the patient with retrograde flow (against the native cardiac output), and oxygen delivery to the upper body may be impeded.
Axillary cannulation, in which the return cannula is placed directly into the axillary artery to provide antegrade flow, has been used recently in patients with pulmonary hypertension or right ventricular failure.22
Venovenous ECMO
Venovenous ECMO provides complete or partial support to the lungs and is the configuration of choice in isolated respiratory failure when cardiac function is preserved. It takes deoxygenated blood from the central venous system—either the femoral vein or internal jugular vein—and returns oxygenated blood to the venous circulation directed into the right atrium. It can be delivered by different cannula configurations based on the patient’s size and clinical requirements.
In the past, the most commonly used configuration was the femoral-atrial, in which the drainage cannula was placed in the femoral vein with the tip advanced to the level of the diaphragm in the inferior vena cava, and the return cannula was placed in the right internal jugular vein with its tip at the junction of the superior vena cava and right atrium. In this configuration, some of the oxygenated blood delivered by the superior vena cava cannula reaches the inferior vena cava cannula, creating a “shunt,” also known as “recirculation.”
Currently, a double-lumen cannula is preferred. This type of cannula is placed in the right internal jugular vein with the tip advanced to the inferior vena cava so that blood is drained through one lumen from both the inferior and superior vena cavas and returned via the other lumen with the jet directed over the tricuspid valve. Advantages of this system are that as it delivers more oxygen to the pulmonary arteries it reduces recirculation, it requires only a single cannula to be inserted, and it facilitates ambulation and rehabilitation in patients requiring long-term ECMO.
A newer double-lumen cannula designed to drain venous blood from the right atrium and reinfuse it directly into the pulmonary artery may provide an alternative for patients with right ventricular failure.
Extracorporeal removal of carbon dioxide
ECMO can remove carbon dioxide in patients with hypercapneic respiratory failure. Early technology used a variation of venovenous ECMO with very low blood flow rates through the pump, which allowed use of smaller cannulas while efficiently removing carbon dioxide.23
Since then, a pumpless extracorporeal lung-assist device has been developed that uses an arteriovenous configuration with two smaller cannulas inserted into the femoral artery and vein (Novalung, Germany).24 Lacking a pump, it avoids the complications associated with pumps such as hemolysis and clotting. It effectively removes carbon dioxide and helps reduce the frequency and intensity of mechanical ventilation. Since the flow is driven by the patient’s arteriovenous pressure gradient, good cardiac output is a prerequisite for its use.
A portable low-blood-flow machine that uses a very small (ie, 15-F) catheter in the venovenous configuration is under investigation (Hemolung RAS, Alung Technologies).
WHO CAN BENEFIT FROM ECMO?
Although evidence to support the routine use of ECMO is limited, tools and guidelines have been developed to help clinicians decide if a patient might benefit from it. Indications for and contraindications to ECMO are shown in Table 2.
The Extracorporeal Life Support Organization recommends considering ECMO if the predicted risk of death is greater than 50% without it, and says ECMO is indicated if the predicted risk exceeds 80%. A scoring system has been developed to help predict the risk of death in patients on ECMO.14 This system has been validated using a historical cohort of patients, and current studies are ongoing for prospective validation.
Many centers are now using ECMO as a salvage therapy in patients with severe respiratory failure when conventional mechanical ventilation and adjunctive therapies such as neuromuscular blockade, inhaled nitric oxide, steroids, prone positioning, and high-frequency oscillation therapy fail to improve gas exchange.25,26
ECMO is also indicated in hypercapneic respiratory failure secondary to status asthmaticus and exacerbation of chronic obstructive pulmonary disease, permissive hypercapnea with a Paco2 greater than 80 mm Hg, or inability to achieve safe inflation pressures with plateau pressures of 30 cm H2O or higher, refractory to conventional therapy.27
Sometimes, delay in referral leads to irreversible ventilator-induced lung injury due to intense mechanical ventilation, thus limiting the utility of ECMO.8 Early referral should be considered if the patient does not improve after a few days on optimal ventilator settings. In centers where this technology is not available, referral to the nearest ECMO center should be considered. A list of certified ECMO centers is available at www.elso.org/Members/CenterDirectory.aspx.
Contraindications to ECMO
Advanced age, comorbid conditions such as malignancy, nonpulmonary organ dysfunction (including complications of critical illness), and immunodeficiency or pharmacologic immune suppression have been associated with poor outcomes in ECMO patients.28 Severe aortic incompetence and aortic dissection are contraindications, since ventricular end-diastolic pressure can be increased with resultant ventricular distention, compromised myocardial oxygenation, and worsening of left heart failure.
ECMO is increasingly being used in situations in which it was previously considered contraindicated. Pregnant and postpartum patients with cardiorespiratory failure were previously not considered for ECMO because of a possible increased risk of coagulopathy and complications. However, a recent review showed that the outcomes of ECMO in pregnancy and postpartum were similar to those in nonpregnant patients, and the risk of catastrophic bleeding was minor.29
Similarly, ECMO is also being used increasingly in posttrauma patients and patients with other bleeding risks.30
Morbid obesity was once considered a contraindication because of difficulty in cannulation, but with newer types of cannulas, even patients with a body mass index greater than 60 kg/m2 are receiving ECMO.31
HOW DO YOU DO IT?
Figures 3 and 4 depict clinical decision-making in starting and weaning from ECMO in respiratory failure and cardiogenic shock, respectively.
Management of patients on ECMO
Appropriate patient selection and initiation of ECMO are only the beginning of a tough journey. Successful management requires minimizing lung injury from mechanical ventilation, careful monitoring of anticoagulation, and instituting adequate physical therapy, including ambulation when possible (Table 3).
Initial ECMO settings and monitoring
The cannulas for venovenous ECMO are frequently inserted under fluoroscopic or transesophageal echocardiographic guidance, whereas venoarterial ECMO cannulation does not require imaging and can be performed at the bedside in the intensive care unit or operating room.
The initial ECMO settings are titrated according to the patient’s hemodynamic and respiratory needs. There are three main variables: blood flow, fraction of oxygen in the sweep gas, and sweep gas flow rate. These are adjusted to achieve desirable levels of oxygen and carbon dioxide in the blood.
Blood flow is determined by the revolutions per minute of the pump, preload, and afterload of the circuit. Common patient conditions that may reduce flow are systemic hypertension, hypovolemia, cardiac tamponade, and tension pneumothorax, depending on the modality. In addition, mechanical factors such as clots in the oxygenator or kinks in the circuit can increase resistance and reduce flow. Resistance to flow is directly proportional to cannula lengths and inversely proportional to cannula radius to the fourth power. The greater the flow, the greater the oxygen delivery.
Fraction of oxygen in the sweep gas. The oxygenator has a gas blender that mixes air and oxygen and allows for a range of oxygen concentrations. Increases in fraction of oxygen increase the partial pressure of oxygen in the blood.
Sweep gas flow rate. Venous blood in the extracorporeal circuit is exposed to fresh gas (or sweep gas) that oxygenates the blood and removes carbon dioxide by diffusion. Increasing the sweep gas flow rate results in greater carbon dioxide elimination from the blood.
Laboratory monitoring. During ECMO, the following values are monitored frequently:
- Arterial blood gases
- Blood gases in the ECMO circuit before and after going through the oxygenator— to monitor the efficacy of the oxygenator membrane
- Lactic acid—to monitor for tissue hypoxia
- Plasma free hemoglobin (a marker of hemolysis)—to monitor for hemolysis.
Mechanical ventilation on ECMO
Low tidal volume ventilation greatly reduces the risk of death in patients on ECMO by reducing ventilator-induced lung injury. Proponents of ECMO believe that ECMO provides “lung rest,” and thus it is imperative that lung-protective ventilation strategies be followed in patients on ECMO.8 In most cases, after ECMO is started, low tidal volume ventilation (6 mL/kg) is possible and should be used—or even very low tidal volume ventilation (3–6 mL/kg).32,33 Many cases have also been described in which patients have been safely extubated while on ECMO to prevent ventilator-induced lung injury.34,35
If hypoxemia persists
Despite full support with venovenous ECMO, some patients remain hypoxemic due to inadequate blood flow to match metabolic demands, eg, patients with morbid obesity or severe sepsis and fever. The physician should ensure there is no recirculation, maximize blood flow, optimize the hematocrit to increase oxygen delivery, and consider ways to decrease oxygen consumption, including sedation, paralysis, and hypothermia.
Recirculation can be calculated by measuring the oxygen saturation of the blood in the ECMO machine before and after it goes through the oxygenator, and also in the central venous blood. Recirculation has been reduced by using double-lumen cannulas but can also be reduced by manipulation of the reinfusion cannula or increasing the distance between drainage and reinfusion ports in other configurations of venovenous ECMO.
Expert opinion suggests that oxygen saturation of 86% or more and Pao2 of 55 mm Hg or more in patients on venovenous ECMO are sufficient to prevent hypoxia-related end-organ injury.36 Venoarterial ECMO should be considered in patients on venovenous ECMO with refractory hypoxemia with the above measures.
Harlequin syndrome is characterized by upper body hypoxia resulting in cerebral hypoxemia due to poorly oxygenated blood in the coronary and cerebral circulations, especially in patients on peripheral venoarterial ECMO. It can be detected by sampling the blood in the arm (where the oxygen isn’t going) instead of the leg (where the oxygen is going), and it can be corrected by adjusting the Fio2, using positive end-expiratory pressure, or both to increase oxygenation. If ventilator settings do not improve this syndrome, the arterial cannulation site can be switched from the femoral artery to the axillary or carotid artery.
Alternatively, a mixed-configuration venoarterial-venous ECMO can also be created, in which a portion of arterialized blood from the arterial outflow cannula is diverted via the right internal jugular artery to the right heart. This enriches the blood traveling through the pulmonary circulation and to the left ventricle to provide better oxygen delivery to the coronary and cerebral circulations.
Anticoagulation monitoring and transfusions
Anticoagulation is necessary to maintain a clot-free and functional circuit. Most clots develop in the oxygenator membrane, where they can prevent optimal gas exchange and, rarely, lead to embolization to the systemic circulation. However, reports have suggested that anticoagulation can be held for short periods on ECMO if necessary.
Unfractionated heparin is usually used for anticoagulation. Commonly used tests to monitor anticoagulation are the augmented partial thromboplastin time, activated clotting time, and anti-factor Xa levels. Lately, thromboelastography analysis is being used to comprehensively monitor various components of the coagulation cascade.37 Anticoagulation is usually tailored to whether there are clots in the circuit, coagulopathy, and bleeding while on ECMO.38
Traditionally, blood products were used liberally during ECMO to maintain a normal hematocrit and improve oxygen delivery, although recent data suggest that outcomes may be similar with conservative use of blood products.39,40
Fluid management on ECMO
ECMO patients are fluid-overloaded due to a profound inflammatory response, cardiac failure, or both. Studies have shown that conservative fluid management improve lung function and shortens the duration of mechanical ventilation and intensive care in patients with lung injury.41 Hence, the patient’s net fluid balance should be kept negative, provided renal and hemodynamic parameters remain stable.
There is a high incidence of acute kidney injury in ECMO patients, and fluid overload is one of the main indications for renal replacement therapy.42 Continuous renal replacement therapy can be provided either by an in-line hemofilter or by incorporating a standard continuous renal replacement therapy machine into the ECMO circuit. There are no studies comparing the efficacy of these techniques, but they allow for rapid improvement in fluid balance and electrolyte disturbances and are commonly used in ECMO patients.42,43
Physical rehabilitation and ambulation on ECMO
Physical rehabilitation in mechanically ventilated patients has been shown to reduce ventilator days and stay in the intensive care unit.44 With the use of internal jugular double-lumen cannulas for venovenous ECMO and improvement in durability of the ECMO circuit, several centers are implementing physical rehabilitation and ambulation for patients while on ECMO. Current data suggest that physical therapy is safe for patients receiving ECMO and may accelerate the weaning process and shorten length of stay in the hospital after ECMO.45,46 Aggressive rehabilitation is especially beneficial in patients awaiting lung transplant and may improve posttransplant recovery and outcomes.47
Weaning from ECMO
There are no standard guidelines for weaning from venovenous or venoarterial ECMO. Once the underlying condition for which ECMO was initiated has improved, weaning can begin by reducing the blood flow rate, the flow rate of the sweep gas, or both.
Weaning from venovenous ECMO should be started when there is improvement in lung compliance, tidal volumes, and oxygenation. Once the circuit flow rate is reduced to less than 3 L/minute, ventilator settings are adjusted to standard lung-protective settings. ECMO support is gradually decreased by reducing the flow rate of sweep gas to less than 2 L/minute. If tidal volumes, respiratory rate, and gas exchange remain adequate after approximately 2 to 4 hours on a low rate of sweep gas, the patient can be weaned off the venovenous ECMO circuit.
Weaning from venoarterial ECMO should be considered when there is myocardial recovery with improved pulse pressure and contractility on echocardiography. This is done by reducing flow rates in increments of 0.5 to 2 L/minute over 24 to 36 hours and monitoring mean arterial pressures, central venous pressure, and myocardial contractility. When acceptable, patients are mostly weaned in a surgical setting. Prolonged periods on a low rate of blood flow are avoided to prevent thrombus formation in the circuit.
COMPLICATIONS OF ECMO
ECMO use can be associated with a myriad of patient and mechanical complications.
Hemorrhage is the most common complication encountered in ECMO, occurring in approximately 43% of patients.29 It occurs most frequently from cannulation and surgical sites. Although rare, potentially life-threatening pulmonary hemorrhage (including bleeding at the tracheostomy site), intracranial hemorrhage, and gastrointestinal hemorrhage have also been reported.30
Infections, including new infection and worsening sepsis in patients with acute respiratory distress syndrome secondary to infection, are common in patients on ECMO.48
Renal failure secondary to acute tubular necrosis requiring hemodialysis has been reported to occur in 13% of patients on ECMO.30
Other complications of concern, especially in patients on venoarterial ECMO, are lower limb ischemia and thromboembolism associated with site of cannulation and direction of blood flow.49 Mechanical complications include inappropriate placement of the cannula leading to insufficient oxygenation, injury to vessel walls, and rarely myocardial wall rupture; thrombus formation within the circuit causing failure of the oxygenator and sometimes, pulmonary or systemic embolism; and air embolism from the circuit.36
NOT SUITED FOR ALL
Despite limited data to support its use, there has been a recent increase in utilization of ECMO to support critically ill adult patients with cardiopulmonary failure. ECMO support is not suited for all patients. Careful selection of patients should be done to optimize resource utilization and provide the best opportunity for recovery or transplant.
Extracorporeal membrance oxygenation (ECMO) provides temporary cardiorespiratory support for patients with severe respiratory or cardiac failure refractory to conventional therapy.1 It can be configured to provide oxygen, remove carbon dioxide, support perfusion, or all of the above. It may provide a bridge to recovery in patients with acute cardiopulmonary failure or to heart or lung transplant.
Developed in the 1970s, ECMO has proven effective and is widely used in children with respiratory and cardiopulmonary failure.2 However, it remained little used in adults, as early randomized trials showed higher rates of complications in adults who received it and no survival advantage.3,4 Proponents of using it in adult patients believe that these poor outcomes were at least partially due to limited training, intensive anticoagulation, and excessive volume and pressure during mechanical ventilation. Although ECMO technology has improved substantially in the last decade and survival rates have improved (www.elso.org), evidence to support its routine use in adults remains limited.
Nevertheless, about 14,000 adult patients received ECMO between 1990 and 2014, with a rate of survival to discharge of 57% for those in respiratory failure and 41% for those in cardiac failure.5 Its use increased 433% in the United States from 2006 to 2011.6
A national survey of critical care physicians and trainees in the United States found they had limited knowledge about ECMO technology and wanted to include specific educational objectives about it in their training.7
This article summarizes the principles of ECMO, including practical aspects such as patient selection, monitoring, and complications.
LIMITED EVIDENCE OF BENEFIT FROM CONTROLLED TRIALS
There is limited evidence from randomized controlled trials that ECMO is beneficial in adults.
In acute respiratory failure, the first randomized trial of ECMO in adults was conducted in 1979 in multiple medical centers.3 The survival rate was no higher with ECMO than with mechanical ventilation alone, and complication rates were very high.
Similarly, Morris et al4 performed a single-center trial comparing pressure-controlled inverse-ratio ventilation and extracorporeal carbon dioxide removal in patients with acute respiratory distress syndrome, which showed no survival benefit.
After these two early trials, ECMO was largely abandoned, and not until 2009 did a multicenter randomized trial in acute respiratory distress syndrome8 rejuvenate interest in its use. Although the trial did not conclusively prove that ECMO was more effective than conventional mechanical ventilation, the findings supported early referral to tertiary care centers with ECMO expertise, and the survival rate was substantially higher than in previous studies. A concise summary of randomized trials and retrospective studies utilizing ECMO in respiratory failure is shown in Table 1.8–14
During the global pandemic of influenza H1N1 in 2009–2010, several centers reported survival benefits from ECMO in patients with severe acute respiratory distress syndrome secondary to influenza.9–12,15–19 Two retrospective case-control studies reported lower mortality rates when H1N1 patients were transferred to ECMO centers10 and among younger patients with H1N1 who received ECMO.12
Ongoing trials (ClinicalTrials.gov identifier NCT01470703) may provide definitive evidence for the effectiveness of ECMO as a rescue therapy in acute respiratory distress syndrome.
In cardiogenic shock, single-center retrospective and observational studies have reported better outcomes for patients who received ECMO for cardiogenic shock secondary to myocardial infarction, pulmonary embolism, sepsis-related cardiomyopathy, and even extracorporeal cardiopulmonary resuscitation.20
WHAT IS ECMO?
In ECMO, venous blood is shunted through a machine to add oxygen, remove carbon dioxide, and regulate temperature (Figure 1). The components of an ECMO circuit are as follows:
- Blood pump
- Membrane oxygenator
- Gas mixer
- Cannulas
- Heater/cooler
- Console.
TWO BASIC CONFIGURATIONS
Two basic ECMO configurations are used in adults: venoarterial and venovenous,21 although combinations of the two—hybrid configurations—are sometimes used (Figure 2).
Venoarterial ECMO
Venoarterial ECMO provides complete or partial support to the heart and lungs and is the configuration of choice in patients with isolated cardiac failure that is refractory to other treatments. It takes deoxygenated blood from the venous system and returns oxygenated blood to the arterial circulation.
In the central venoarterial configuration, the intake cannula is most often surgically placed in the right atrium and the return cannula is placed in the proximal ascending aorta.
In the peripheral femoral configuration, the drainage cannula is placed in the femoral vein and advanced to the right atrium, and the return cannula is placed in either the ipsilateral or contralateral femoral artery. However, this configuration provides the patient with retrograde flow (against the native cardiac output), and oxygen delivery to the upper body may be impeded.
Axillary cannulation, in which the return cannula is placed directly into the axillary artery to provide antegrade flow, has been used recently in patients with pulmonary hypertension or right ventricular failure.22
Venovenous ECMO
Venovenous ECMO provides complete or partial support to the lungs and is the configuration of choice in isolated respiratory failure when cardiac function is preserved. It takes deoxygenated blood from the central venous system—either the femoral vein or internal jugular vein—and returns oxygenated blood to the venous circulation directed into the right atrium. It can be delivered by different cannula configurations based on the patient’s size and clinical requirements.
In the past, the most commonly used configuration was the femoral-atrial, in which the drainage cannula was placed in the femoral vein with the tip advanced to the level of the diaphragm in the inferior vena cava, and the return cannula was placed in the right internal jugular vein with its tip at the junction of the superior vena cava and right atrium. In this configuration, some of the oxygenated blood delivered by the superior vena cava cannula reaches the inferior vena cava cannula, creating a “shunt,” also known as “recirculation.”
Currently, a double-lumen cannula is preferred. This type of cannula is placed in the right internal jugular vein with the tip advanced to the inferior vena cava so that blood is drained through one lumen from both the inferior and superior vena cavas and returned via the other lumen with the jet directed over the tricuspid valve. Advantages of this system are that as it delivers more oxygen to the pulmonary arteries it reduces recirculation, it requires only a single cannula to be inserted, and it facilitates ambulation and rehabilitation in patients requiring long-term ECMO.
A newer double-lumen cannula designed to drain venous blood from the right atrium and reinfuse it directly into the pulmonary artery may provide an alternative for patients with right ventricular failure.
Extracorporeal removal of carbon dioxide
ECMO can remove carbon dioxide in patients with hypercapneic respiratory failure. Early technology used a variation of venovenous ECMO with very low blood flow rates through the pump, which allowed use of smaller cannulas while efficiently removing carbon dioxide.23
Since then, a pumpless extracorporeal lung-assist device has been developed that uses an arteriovenous configuration with two smaller cannulas inserted into the femoral artery and vein (Novalung, Germany).24 Lacking a pump, it avoids the complications associated with pumps such as hemolysis and clotting. It effectively removes carbon dioxide and helps reduce the frequency and intensity of mechanical ventilation. Since the flow is driven by the patient’s arteriovenous pressure gradient, good cardiac output is a prerequisite for its use.
A portable low-blood-flow machine that uses a very small (ie, 15-F) catheter in the venovenous configuration is under investigation (Hemolung RAS, Alung Technologies).
WHO CAN BENEFIT FROM ECMO?
Although evidence to support the routine use of ECMO is limited, tools and guidelines have been developed to help clinicians decide if a patient might benefit from it. Indications for and contraindications to ECMO are shown in Table 2.
The Extracorporeal Life Support Organization recommends considering ECMO if the predicted risk of death is greater than 50% without it, and says ECMO is indicated if the predicted risk exceeds 80%. A scoring system has been developed to help predict the risk of death in patients on ECMO.14 This system has been validated using a historical cohort of patients, and current studies are ongoing for prospective validation.
Many centers are now using ECMO as a salvage therapy in patients with severe respiratory failure when conventional mechanical ventilation and adjunctive therapies such as neuromuscular blockade, inhaled nitric oxide, steroids, prone positioning, and high-frequency oscillation therapy fail to improve gas exchange.25,26
ECMO is also indicated in hypercapneic respiratory failure secondary to status asthmaticus and exacerbation of chronic obstructive pulmonary disease, permissive hypercapnea with a Paco2 greater than 80 mm Hg, or inability to achieve safe inflation pressures with plateau pressures of 30 cm H2O or higher, refractory to conventional therapy.27
Sometimes, delay in referral leads to irreversible ventilator-induced lung injury due to intense mechanical ventilation, thus limiting the utility of ECMO.8 Early referral should be considered if the patient does not improve after a few days on optimal ventilator settings. In centers where this technology is not available, referral to the nearest ECMO center should be considered. A list of certified ECMO centers is available at www.elso.org/Members/CenterDirectory.aspx.
Contraindications to ECMO
Advanced age, comorbid conditions such as malignancy, nonpulmonary organ dysfunction (including complications of critical illness), and immunodeficiency or pharmacologic immune suppression have been associated with poor outcomes in ECMO patients.28 Severe aortic incompetence and aortic dissection are contraindications, since ventricular end-diastolic pressure can be increased with resultant ventricular distention, compromised myocardial oxygenation, and worsening of left heart failure.
ECMO is increasingly being used in situations in which it was previously considered contraindicated. Pregnant and postpartum patients with cardiorespiratory failure were previously not considered for ECMO because of a possible increased risk of coagulopathy and complications. However, a recent review showed that the outcomes of ECMO in pregnancy and postpartum were similar to those in nonpregnant patients, and the risk of catastrophic bleeding was minor.29
Similarly, ECMO is also being used increasingly in posttrauma patients and patients with other bleeding risks.30
Morbid obesity was once considered a contraindication because of difficulty in cannulation, but with newer types of cannulas, even patients with a body mass index greater than 60 kg/m2 are receiving ECMO.31
HOW DO YOU DO IT?
Figures 3 and 4 depict clinical decision-making in starting and weaning from ECMO in respiratory failure and cardiogenic shock, respectively.
Management of patients on ECMO
Appropriate patient selection and initiation of ECMO are only the beginning of a tough journey. Successful management requires minimizing lung injury from mechanical ventilation, careful monitoring of anticoagulation, and instituting adequate physical therapy, including ambulation when possible (Table 3).
Initial ECMO settings and monitoring
The cannulas for venovenous ECMO are frequently inserted under fluoroscopic or transesophageal echocardiographic guidance, whereas venoarterial ECMO cannulation does not require imaging and can be performed at the bedside in the intensive care unit or operating room.
The initial ECMO settings are titrated according to the patient’s hemodynamic and respiratory needs. There are three main variables: blood flow, fraction of oxygen in the sweep gas, and sweep gas flow rate. These are adjusted to achieve desirable levels of oxygen and carbon dioxide in the blood.
Blood flow is determined by the revolutions per minute of the pump, preload, and afterload of the circuit. Common patient conditions that may reduce flow are systemic hypertension, hypovolemia, cardiac tamponade, and tension pneumothorax, depending on the modality. In addition, mechanical factors such as clots in the oxygenator or kinks in the circuit can increase resistance and reduce flow. Resistance to flow is directly proportional to cannula lengths and inversely proportional to cannula radius to the fourth power. The greater the flow, the greater the oxygen delivery.
Fraction of oxygen in the sweep gas. The oxygenator has a gas blender that mixes air and oxygen and allows for a range of oxygen concentrations. Increases in fraction of oxygen increase the partial pressure of oxygen in the blood.
Sweep gas flow rate. Venous blood in the extracorporeal circuit is exposed to fresh gas (or sweep gas) that oxygenates the blood and removes carbon dioxide by diffusion. Increasing the sweep gas flow rate results in greater carbon dioxide elimination from the blood.
Laboratory monitoring. During ECMO, the following values are monitored frequently:
- Arterial blood gases
- Blood gases in the ECMO circuit before and after going through the oxygenator— to monitor the efficacy of the oxygenator membrane
- Lactic acid—to monitor for tissue hypoxia
- Plasma free hemoglobin (a marker of hemolysis)—to monitor for hemolysis.
Mechanical ventilation on ECMO
Low tidal volume ventilation greatly reduces the risk of death in patients on ECMO by reducing ventilator-induced lung injury. Proponents of ECMO believe that ECMO provides “lung rest,” and thus it is imperative that lung-protective ventilation strategies be followed in patients on ECMO.8 In most cases, after ECMO is started, low tidal volume ventilation (6 mL/kg) is possible and should be used—or even very low tidal volume ventilation (3–6 mL/kg).32,33 Many cases have also been described in which patients have been safely extubated while on ECMO to prevent ventilator-induced lung injury.34,35
If hypoxemia persists
Despite full support with venovenous ECMO, some patients remain hypoxemic due to inadequate blood flow to match metabolic demands, eg, patients with morbid obesity or severe sepsis and fever. The physician should ensure there is no recirculation, maximize blood flow, optimize the hematocrit to increase oxygen delivery, and consider ways to decrease oxygen consumption, including sedation, paralysis, and hypothermia.
Recirculation can be calculated by measuring the oxygen saturation of the blood in the ECMO machine before and after it goes through the oxygenator, and also in the central venous blood. Recirculation has been reduced by using double-lumen cannulas but can also be reduced by manipulation of the reinfusion cannula or increasing the distance between drainage and reinfusion ports in other configurations of venovenous ECMO.
Expert opinion suggests that oxygen saturation of 86% or more and Pao2 of 55 mm Hg or more in patients on venovenous ECMO are sufficient to prevent hypoxia-related end-organ injury.36 Venoarterial ECMO should be considered in patients on venovenous ECMO with refractory hypoxemia with the above measures.
Harlequin syndrome is characterized by upper body hypoxia resulting in cerebral hypoxemia due to poorly oxygenated blood in the coronary and cerebral circulations, especially in patients on peripheral venoarterial ECMO. It can be detected by sampling the blood in the arm (where the oxygen isn’t going) instead of the leg (where the oxygen is going), and it can be corrected by adjusting the Fio2, using positive end-expiratory pressure, or both to increase oxygenation. If ventilator settings do not improve this syndrome, the arterial cannulation site can be switched from the femoral artery to the axillary or carotid artery.
Alternatively, a mixed-configuration venoarterial-venous ECMO can also be created, in which a portion of arterialized blood from the arterial outflow cannula is diverted via the right internal jugular artery to the right heart. This enriches the blood traveling through the pulmonary circulation and to the left ventricle to provide better oxygen delivery to the coronary and cerebral circulations.
Anticoagulation monitoring and transfusions
Anticoagulation is necessary to maintain a clot-free and functional circuit. Most clots develop in the oxygenator membrane, where they can prevent optimal gas exchange and, rarely, lead to embolization to the systemic circulation. However, reports have suggested that anticoagulation can be held for short periods on ECMO if necessary.
Unfractionated heparin is usually used for anticoagulation. Commonly used tests to monitor anticoagulation are the augmented partial thromboplastin time, activated clotting time, and anti-factor Xa levels. Lately, thromboelastography analysis is being used to comprehensively monitor various components of the coagulation cascade.37 Anticoagulation is usually tailored to whether there are clots in the circuit, coagulopathy, and bleeding while on ECMO.38
Traditionally, blood products were used liberally during ECMO to maintain a normal hematocrit and improve oxygen delivery, although recent data suggest that outcomes may be similar with conservative use of blood products.39,40
Fluid management on ECMO
ECMO patients are fluid-overloaded due to a profound inflammatory response, cardiac failure, or both. Studies have shown that conservative fluid management improve lung function and shortens the duration of mechanical ventilation and intensive care in patients with lung injury.41 Hence, the patient’s net fluid balance should be kept negative, provided renal and hemodynamic parameters remain stable.
There is a high incidence of acute kidney injury in ECMO patients, and fluid overload is one of the main indications for renal replacement therapy.42 Continuous renal replacement therapy can be provided either by an in-line hemofilter or by incorporating a standard continuous renal replacement therapy machine into the ECMO circuit. There are no studies comparing the efficacy of these techniques, but they allow for rapid improvement in fluid balance and electrolyte disturbances and are commonly used in ECMO patients.42,43
Physical rehabilitation and ambulation on ECMO
Physical rehabilitation in mechanically ventilated patients has been shown to reduce ventilator days and stay in the intensive care unit.44 With the use of internal jugular double-lumen cannulas for venovenous ECMO and improvement in durability of the ECMO circuit, several centers are implementing physical rehabilitation and ambulation for patients while on ECMO. Current data suggest that physical therapy is safe for patients receiving ECMO and may accelerate the weaning process and shorten length of stay in the hospital after ECMO.45,46 Aggressive rehabilitation is especially beneficial in patients awaiting lung transplant and may improve posttransplant recovery and outcomes.47
Weaning from ECMO
There are no standard guidelines for weaning from venovenous or venoarterial ECMO. Once the underlying condition for which ECMO was initiated has improved, weaning can begin by reducing the blood flow rate, the flow rate of the sweep gas, or both.
Weaning from venovenous ECMO should be started when there is improvement in lung compliance, tidal volumes, and oxygenation. Once the circuit flow rate is reduced to less than 3 L/minute, ventilator settings are adjusted to standard lung-protective settings. ECMO support is gradually decreased by reducing the flow rate of sweep gas to less than 2 L/minute. If tidal volumes, respiratory rate, and gas exchange remain adequate after approximately 2 to 4 hours on a low rate of sweep gas, the patient can be weaned off the venovenous ECMO circuit.
Weaning from venoarterial ECMO should be considered when there is myocardial recovery with improved pulse pressure and contractility on echocardiography. This is done by reducing flow rates in increments of 0.5 to 2 L/minute over 24 to 36 hours and monitoring mean arterial pressures, central venous pressure, and myocardial contractility. When acceptable, patients are mostly weaned in a surgical setting. Prolonged periods on a low rate of blood flow are avoided to prevent thrombus formation in the circuit.
COMPLICATIONS OF ECMO
ECMO use can be associated with a myriad of patient and mechanical complications.
Hemorrhage is the most common complication encountered in ECMO, occurring in approximately 43% of patients.29 It occurs most frequently from cannulation and surgical sites. Although rare, potentially life-threatening pulmonary hemorrhage (including bleeding at the tracheostomy site), intracranial hemorrhage, and gastrointestinal hemorrhage have also been reported.30
Infections, including new infection and worsening sepsis in patients with acute respiratory distress syndrome secondary to infection, are common in patients on ECMO.48
Renal failure secondary to acute tubular necrosis requiring hemodialysis has been reported to occur in 13% of patients on ECMO.30
Other complications of concern, especially in patients on venoarterial ECMO, are lower limb ischemia and thromboembolism associated with site of cannulation and direction of blood flow.49 Mechanical complications include inappropriate placement of the cannula leading to insufficient oxygenation, injury to vessel walls, and rarely myocardial wall rupture; thrombus formation within the circuit causing failure of the oxygenator and sometimes, pulmonary or systemic embolism; and air embolism from the circuit.36
NOT SUITED FOR ALL
Despite limited data to support its use, there has been a recent increase in utilization of ECMO to support critically ill adult patients with cardiopulmonary failure. ECMO support is not suited for all patients. Careful selection of patients should be done to optimize resource utilization and provide the best opportunity for recovery or transplant.
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012; 38:210–220.
- Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: history, development and current status. World J Crit Care Med 2013; 2:29–39.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Extracorporeal Life Support Organization. ECLS registry report. International Summary. January 2016. https://www.elso.org/Registry.aspx. Accessed March 17, 2016.
- Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015; 61:31–36.
- Sharma N, Wille K, Bellot S, Brodie D, Diaz-Guzman E. Role of extracorporeal membrane oxygenation in management of refractory ARDS in the intensive care unit: a national survey on perspectives of the adult critical care physicians and trainees. Chest 2014. http://journal.publications.chestnet.org/article.aspx?articleid=1913336. Accessed March 17, 2016.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302:1888–1895.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011; 37:1447–1457.
- Pham T, Combes A, Roze H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39; 532:1704–1713.
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189:1374–1382.
- Chan KK, Lee KL, Lam PK, Law KI, Joynt GM, Yan WW. Hong Kong's experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1). Hong Kong Med J 2010; 16:447–454.
- Freed DH, Henzler D, White CW, et al; Canadian Critical Care Trials Group. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth 2010; 57:240–247.
- Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 2011; 37:648–654.
- Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care 2011; 56:941–946.
- Kumar A, Zarychanski R, Pinto R, et al; Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879.
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014; 63:2769–2778.
- Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008; 17(suppl 4):S41–S47.
- Hysi I, Fabre O, Renaut C, Guesnier L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg 2014; 29:268–269.
- Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs 1979; 2:282–283.
- Liebold A, Philipp A, Kaiser M, Merk J, Schmid FX, Birnbaum DE. Pumpless extracorporeal lung assist using an arterio-venous shunt. Applications and limitations. Minerva Anestesiol 2002; 68:387–391.
- Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013; 59:202–210.
- Shekar K, Davies AR, Mullany DV, Tiruvoipati R, Fraser JF. To ventilate, oscillate, or cannulate? J Crit Care 2013; 28:655–662.
- Mikkelsen ME, Woo YJ, Sager JS, Fuchs BD, Christie JD. Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J 2009; 55:47–52.
- Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care 2013; 58:1038–1052.
- Sharma NS, Wille KM, Bellot SC, Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J 2015; 61:110–114.
- Ried M, Bein T, Philipp A, et al. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care 2013; 17:R110.
- Al-Soufi S, Buscher H, Nguyen ND, Rycus P, Nair P. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med 2013; 39:1995–2002.
- Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 2014; 11:956–961.
- Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014; 18:203.
- Bein T, Wittmann S, Philipp A, Nerlich M, Kuehnel T, Schlitt HJ. Successful extubation of an "unweanable" patient with severe ankylosing spondylitis (Bechterew's disease) using a pumpless extracorporeal lung assist. Intensive Care Med 2008; 34:2313–2314.
- Anton-Martin P, Thompson MT, Sheeran PD, Fischer AC, Taylor D, Thomas JA. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatr Crit Care Med 2014; 15:861–869.
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth 2010; 24:164–172.
- Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol 1995; 27:137–145.
- Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013; 59:63–68.
- Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015; 99:590–595.
- Voelker MT, Busch T, Bercker S, Fichtner F, Kaisers UX, Laudi S. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artif Organs 2015; 39:374–378.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J 2012; 58:407–414.
- Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2012; 7:1328–1336.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18:R38.
- Thiagarajan RR, Teele SA, Teele KP, Beke DM. Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med 2012; 5:47–52.
- Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013; 145:862–868.
- Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis 1999; 28:828–833.
- Lafç G, Budak AB, Yener AÜ, Cicek OF. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ 2014; 23:10–23.
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012; 38:210–220.
- Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: history, development and current status. World J Crit Care Med 2013; 2:29–39.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Extracorporeal Life Support Organization. ECLS registry report. International Summary. January 2016. https://www.elso.org/Registry.aspx. Accessed March 17, 2016.
- Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015; 61:31–36.
- Sharma N, Wille K, Bellot S, Brodie D, Diaz-Guzman E. Role of extracorporeal membrane oxygenation in management of refractory ARDS in the intensive care unit: a national survey on perspectives of the adult critical care physicians and trainees. Chest 2014. http://journal.publications.chestnet.org/article.aspx?articleid=1913336. Accessed March 17, 2016.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302:1888–1895.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011; 37:1447–1457.
- Pham T, Combes A, Roze H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39; 532:1704–1713.
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189:1374–1382.
- Chan KK, Lee KL, Lam PK, Law KI, Joynt GM, Yan WW. Hong Kong's experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1). Hong Kong Med J 2010; 16:447–454.
- Freed DH, Henzler D, White CW, et al; Canadian Critical Care Trials Group. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth 2010; 57:240–247.
- Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 2011; 37:648–654.
- Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care 2011; 56:941–946.
- Kumar A, Zarychanski R, Pinto R, et al; Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879.
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014; 63:2769–2778.
- Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008; 17(suppl 4):S41–S47.
- Hysi I, Fabre O, Renaut C, Guesnier L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg 2014; 29:268–269.
- Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs 1979; 2:282–283.
- Liebold A, Philipp A, Kaiser M, Merk J, Schmid FX, Birnbaum DE. Pumpless extracorporeal lung assist using an arterio-venous shunt. Applications and limitations. Minerva Anestesiol 2002; 68:387–391.
- Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013; 59:202–210.
- Shekar K, Davies AR, Mullany DV, Tiruvoipati R, Fraser JF. To ventilate, oscillate, or cannulate? J Crit Care 2013; 28:655–662.
- Mikkelsen ME, Woo YJ, Sager JS, Fuchs BD, Christie JD. Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J 2009; 55:47–52.
- Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care 2013; 58:1038–1052.
- Sharma NS, Wille KM, Bellot SC, Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J 2015; 61:110–114.
- Ried M, Bein T, Philipp A, et al. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care 2013; 17:R110.
- Al-Soufi S, Buscher H, Nguyen ND, Rycus P, Nair P. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med 2013; 39:1995–2002.
- Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 2014; 11:956–961.
- Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014; 18:203.
- Bein T, Wittmann S, Philipp A, Nerlich M, Kuehnel T, Schlitt HJ. Successful extubation of an "unweanable" patient with severe ankylosing spondylitis (Bechterew's disease) using a pumpless extracorporeal lung assist. Intensive Care Med 2008; 34:2313–2314.
- Anton-Martin P, Thompson MT, Sheeran PD, Fischer AC, Taylor D, Thomas JA. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatr Crit Care Med 2014; 15:861–869.
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth 2010; 24:164–172.
- Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol 1995; 27:137–145.
- Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013; 59:63–68.
- Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015; 99:590–595.
- Voelker MT, Busch T, Bercker S, Fichtner F, Kaisers UX, Laudi S. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artif Organs 2015; 39:374–378.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J 2012; 58:407–414.
- Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2012; 7:1328–1336.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18:R38.
- Thiagarajan RR, Teele SA, Teele KP, Beke DM. Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med 2012; 5:47–52.
- Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013; 145:862–868.
- Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis 1999; 28:828–833.
- Lafç G, Budak AB, Yener AÜ, Cicek OF. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ 2014; 23:10–23.
KEY POINTS
- Two basic configurations of ECMO are used in adults: venoarterial, which can provide cardiac or cardiopulmonary support; and venovenous, which provides respiratory support only.
- ECMO is used in adults who are at very high risk of death without it.
- Because ECMO patients must receive anticoagulation, bleeding is a common complication. Others are infection, renal failure, and thrombosis.
- ECMO may provide “lung rest,” allowing lower tidal volumes and pressures and lower fractions of inspired oxygen to be used in mechanical ventilation, strategies associated with lower mortality rates.