User login
Identifying hyperthyroidism’s psychiatric presentations
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
Identifying hypothyroidism’s psychiatric presentations
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
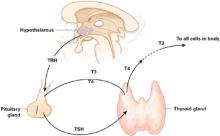
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
Persistent depression? Low libido? Androgen decline may be to blame
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.