User login
Do hormonal contraceptives lead to weight gain?
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
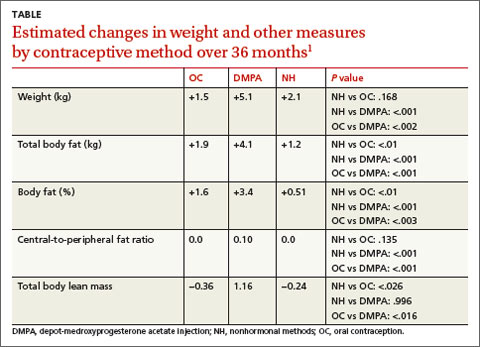
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).
DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).

DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).

DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
Evidence-based answers from the Family Physicians Inquiries Network
PT or cervical collar for cervical radiculopathy?
To shorten recovery time for adults with acute cervical radiculopathy, recommend either physical therapy (PT) and a home exercise plan or a cervical collar and rest.1 Both are more effective than a wait-and-see strategy.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ. 2009;339:b3883.
Illustrative case
James M, a 43-year-old self-employed mechanic, came to see you 2 weeks ago because of neck pain radiating to his right shoulder, arm, forearm, and dorsum of his hand. You diagnosed acute right-sided cervical radiculopathy and prescribed a nonsteroidal anti-inflammatory drug.
Today he’s back in your office, reporting that he has experienced only minimal transient relief. You reassure him that the pain will subside within a few months, but james wants to know if you can give him something to speed up his recovery and enable him to return to work.
Each year in the United States, approximately 85 out of every 100,000 adults develop cervical radiculopathy2—a neurologic condition characterized by dysfunction of a cervical spinal nerve, the roots of the nerve, or both. In addition to pain in the neck and the arm on the affected side, patients often develop sensory loss, loss of motor function, and/or reflex changes in the affected nerve-root distribution.
Most patients respond to conservative measures
A nonsurgical approach is the preferred first-line treatment strategy for cervical radiculopathy.3 The Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders—an international network of experts in a number of specialties—found no evidence that surgery provides better long-term outcomes than more conservative treatment.3 Approximately 80% to 90% of patients respond to a conservative approach, with improvements in pain, function, and mood in 3 to 6 months.4,5
There are numerous conservative therapies for cervical radiculopathy, including oral analgesics, rest, cervical traction, short-term immobilization with a cervical collar, PT, a short course of oral corticosteroids, and perineural steroid injections.4-6 These therapies may be used singly or in combination. Until now, however, no high-quality RCTs compared the efficacy of various nonsurgical treatment modalities for acute cervical radiculopathy—and their effectiveness is still subject to debate.
STUDY SUMMARY: Initially, both Tx modes beat wait-and-see
The study by Kuijper et al1 is the first RCT to compare the effectiveness of PT, cervical collars, and a wait-and-see strategy in alleviating symptoms of cervical radiculopathy. Enrollees (N=205) were men and women ages 18 to 75 years who were referred by general practitioners in 3 Dutch hospitals. All the participants had a diagnosis of cervical radiculopathy confirmed by a neurologist. In addition, all the cases were of recent onset, with symptoms of <1 month’s duration at the time of enrollment. Patients with clinical signs of cord compression and those who had previously been treated with either PT or a cervical collar for this episode were excluded.
The researchers randomized the participants into 3 groups: PT, cervical collar, or control. All the groups were comparable at baseline.
Those in the PT group received twice weekly therapy for 6 weeks, with a focus on mobilizing and stabilizing the cervical spine. They were also taught to perform home exercises and advised to do the exercises daily.
Patients in the cervical collar group were given a semi-hard, snugly fitted collar and instructed to wear it during the day for 3 weeks—and to rest as much as possible. They were weaned from the collar over the course of another 3 weeks.
Participants in the control group were simply told to follow their normal daily routine as much as possible. All 3 groups were permitted to take oral pain medication as needed.
The primary outcome measures were changes over time in neck and arm pain scores, using 2 validated measurement tools: a 100-mm visual analog scale (VAS) and a 100-point neck disability index (NDI). Both tools were used at 3 weeks, 6 weeks, and 6 months. Secondary outcomes were treatment satisfaction (as measured on a 5-point scale), use of opiates, and working status.
By 6 months, differences virtually disappeared
Both the active and passive interventions reduced arm and neck pain faster than the wait-and-see strategy. At 6 weeks, participants in both the PT and cervical collar groups reported a 31-mm reduction in arm pain (P=.007 and .006, respectively), compared with a 19-mm reduction for those in the control group (P=.006). This is a clinically meaningful difference.
The rate of reduction in neck pain over the first 6 weeks was: PT group, 2.4 mm/week, P=.002; cervical collar group, 2.8 mm/week, P=<.001; and control group, 0.9 mm/week. The rate of reduction in the NDI was 1.4 points per week for the control group vs 2.3 points per week for the cervical collar group (P= .024). The PT group fared no better on the NDI measure than the control group. This may reflect the fact that the index predominantly measured disability caused by neck pain, whereas arm pain scores,—which were highest initially—showed the greatest improvement, the authors note.
At 6 months, pain and disability had almost resolved for all the patients, regardless of their treatment group, and secondary outcomes—treatment satisfaction, analgesic use, and working status—were similar for all 3 groups.
WHAT'S NEW: High-quality RCT supports PT and cervical collar
Some investigators have advocated the short-term use of immobilization with either a cervical collar or a cervical pillow during sleep. Until now, however, there was no conclusive evidence about the benefits of this approach.
One earlier RCT (N=493) compared 5 treatment modalities—traction, positioning, collar, placebo tablets, and heat treatment—and found no significant difference in pain and ability to work.7 That trial was done nearly 15 years ago, however, and the investigators did not use validated outcome scales. Therefore, the trial would not meet current RCT standards.
The study we report on here leaves little doubt that the 2 treatments reviewed—PT and cervical collar—provide more rapid relief than a wait-and-see approach.
CAVEATS: Pain meds still needed, unanswered questions remain
Although the cervical collar and PT groups had less pain at 3 and 6 weeks compared with the controls—and all 3 groups showed equal improvement at study’s end—the researchers found little difference in use of analgesics. Data on adherence to treatment was recorded by patients, so treatment adherence may not be completely accurate.8
Patients without severe arm pain or signs of muscle weakness were not included in this study, so we don’t know whether individuals with less severe cervical radiculopathy would benefit from these treatments. What’s more, this study focused only on new cases of acute cervical radiculopathy, and the findings may not apply to patients with chronic, recurrent, or persistent symptoms.
The apparent contradiction in the finding that both immobilization and PT are beneficial does not have a clear scientific explanation. The researchers hypothesize that immobilizing the neck with a collar reduces foraminal root compression and inflammation; this could explain the larger reduction in arm pain compared with neck pain and neck disability found in this study. The mechanism of pain reduction with PT is unclear, although it is probably related to the restoration of the neck musculature’s strength and range of motion.
Cost is another issue. A cervical collar and rest is at least as effective as PT for recent onset cervical radiculopathy, but the collar costs only about $20—far less than the cost of 12 sessions of therapy.
One final caveat: Any patient with persistent or worsening symptoms should undergo additional evaluation, including imaging.
CHALLENGES TO IMPLEMENTATION: Rest is contrary to usual approach
Some physicians may not agree with the recommendation to encourage rest. Indeed, rest and immobilization are contrary to the usual recommendation for musculoskeletal injuries—to resume activity as soon as possible.
Patients might not like wearing a collar for a variety of personal reasons, such as cosmetic appearance or limitations of motion. On the other hand, some patients may feel that their pain is too severe to be able to participate in PT—which may also be too expensive for, or not readily available to, some patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ 2009;339:b3883.-
2. Radhakrishan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990 Brain 1994;117:325-335.
3. Nordin M, Carragee EJ, Hogg-Johnson S, et al. Assessment of neck pain and its associated disorders: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008;33(suppl 4):S101-S122.
4. Saal JS, Saal JA, Yurth EF. Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine 1996;21:1877-1883.
5. Persson LC, Carlsson CA, Carlsson JY. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar: a prospective, randomized study. Spine 1997;22:751-758.
6. Wolff MW, Levine LA. Cervical radiculopathies: conservative approaches to management. Phys Med Rehabil Clin N Am 2002;13:589-608.
7. Levine MJ, Albert TJ, Smith MD. Cervical radiculopathy: diagnosis and nonoperative management. J Am Acad Orthop Surg. 1996;4:305-316.
8. Wainner RS, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine 2003;28:52-62.
To shorten recovery time for adults with acute cervical radiculopathy, recommend either physical therapy (PT) and a home exercise plan or a cervical collar and rest.1 Both are more effective than a wait-and-see strategy.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ. 2009;339:b3883.
Illustrative case
James M, a 43-year-old self-employed mechanic, came to see you 2 weeks ago because of neck pain radiating to his right shoulder, arm, forearm, and dorsum of his hand. You diagnosed acute right-sided cervical radiculopathy and prescribed a nonsteroidal anti-inflammatory drug.
Today he’s back in your office, reporting that he has experienced only minimal transient relief. You reassure him that the pain will subside within a few months, but james wants to know if you can give him something to speed up his recovery and enable him to return to work.
Each year in the United States, approximately 85 out of every 100,000 adults develop cervical radiculopathy2—a neurologic condition characterized by dysfunction of a cervical spinal nerve, the roots of the nerve, or both. In addition to pain in the neck and the arm on the affected side, patients often develop sensory loss, loss of motor function, and/or reflex changes in the affected nerve-root distribution.
Most patients respond to conservative measures
A nonsurgical approach is the preferred first-line treatment strategy for cervical radiculopathy.3 The Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders—an international network of experts in a number of specialties—found no evidence that surgery provides better long-term outcomes than more conservative treatment.3 Approximately 80% to 90% of patients respond to a conservative approach, with improvements in pain, function, and mood in 3 to 6 months.4,5
There are numerous conservative therapies for cervical radiculopathy, including oral analgesics, rest, cervical traction, short-term immobilization with a cervical collar, PT, a short course of oral corticosteroids, and perineural steroid injections.4-6 These therapies may be used singly or in combination. Until now, however, no high-quality RCTs compared the efficacy of various nonsurgical treatment modalities for acute cervical radiculopathy—and their effectiveness is still subject to debate.
STUDY SUMMARY: Initially, both Tx modes beat wait-and-see
The study by Kuijper et al1 is the first RCT to compare the effectiveness of PT, cervical collars, and a wait-and-see strategy in alleviating symptoms of cervical radiculopathy. Enrollees (N=205) were men and women ages 18 to 75 years who were referred by general practitioners in 3 Dutch hospitals. All the participants had a diagnosis of cervical radiculopathy confirmed by a neurologist. In addition, all the cases were of recent onset, with symptoms of <1 month’s duration at the time of enrollment. Patients with clinical signs of cord compression and those who had previously been treated with either PT or a cervical collar for this episode were excluded.
The researchers randomized the participants into 3 groups: PT, cervical collar, or control. All the groups were comparable at baseline.
Those in the PT group received twice weekly therapy for 6 weeks, with a focus on mobilizing and stabilizing the cervical spine. They were also taught to perform home exercises and advised to do the exercises daily.
Patients in the cervical collar group were given a semi-hard, snugly fitted collar and instructed to wear it during the day for 3 weeks—and to rest as much as possible. They were weaned from the collar over the course of another 3 weeks.
Participants in the control group were simply told to follow their normal daily routine as much as possible. All 3 groups were permitted to take oral pain medication as needed.
The primary outcome measures were changes over time in neck and arm pain scores, using 2 validated measurement tools: a 100-mm visual analog scale (VAS) and a 100-point neck disability index (NDI). Both tools were used at 3 weeks, 6 weeks, and 6 months. Secondary outcomes were treatment satisfaction (as measured on a 5-point scale), use of opiates, and working status.
By 6 months, differences virtually disappeared
Both the active and passive interventions reduced arm and neck pain faster than the wait-and-see strategy. At 6 weeks, participants in both the PT and cervical collar groups reported a 31-mm reduction in arm pain (P=.007 and .006, respectively), compared with a 19-mm reduction for those in the control group (P=.006). This is a clinically meaningful difference.
The rate of reduction in neck pain over the first 6 weeks was: PT group, 2.4 mm/week, P=.002; cervical collar group, 2.8 mm/week, P=<.001; and control group, 0.9 mm/week. The rate of reduction in the NDI was 1.4 points per week for the control group vs 2.3 points per week for the cervical collar group (P= .024). The PT group fared no better on the NDI measure than the control group. This may reflect the fact that the index predominantly measured disability caused by neck pain, whereas arm pain scores,—which were highest initially—showed the greatest improvement, the authors note.
At 6 months, pain and disability had almost resolved for all the patients, regardless of their treatment group, and secondary outcomes—treatment satisfaction, analgesic use, and working status—were similar for all 3 groups.
WHAT'S NEW: High-quality RCT supports PT and cervical collar
Some investigators have advocated the short-term use of immobilization with either a cervical collar or a cervical pillow during sleep. Until now, however, there was no conclusive evidence about the benefits of this approach.
One earlier RCT (N=493) compared 5 treatment modalities—traction, positioning, collar, placebo tablets, and heat treatment—and found no significant difference in pain and ability to work.7 That trial was done nearly 15 years ago, however, and the investigators did not use validated outcome scales. Therefore, the trial would not meet current RCT standards.
The study we report on here leaves little doubt that the 2 treatments reviewed—PT and cervical collar—provide more rapid relief than a wait-and-see approach.
CAVEATS: Pain meds still needed, unanswered questions remain
Although the cervical collar and PT groups had less pain at 3 and 6 weeks compared with the controls—and all 3 groups showed equal improvement at study’s end—the researchers found little difference in use of analgesics. Data on adherence to treatment was recorded by patients, so treatment adherence may not be completely accurate.8
Patients without severe arm pain or signs of muscle weakness were not included in this study, so we don’t know whether individuals with less severe cervical radiculopathy would benefit from these treatments. What’s more, this study focused only on new cases of acute cervical radiculopathy, and the findings may not apply to patients with chronic, recurrent, or persistent symptoms.
The apparent contradiction in the finding that both immobilization and PT are beneficial does not have a clear scientific explanation. The researchers hypothesize that immobilizing the neck with a collar reduces foraminal root compression and inflammation; this could explain the larger reduction in arm pain compared with neck pain and neck disability found in this study. The mechanism of pain reduction with PT is unclear, although it is probably related to the restoration of the neck musculature’s strength and range of motion.
Cost is another issue. A cervical collar and rest is at least as effective as PT for recent onset cervical radiculopathy, but the collar costs only about $20—far less than the cost of 12 sessions of therapy.
One final caveat: Any patient with persistent or worsening symptoms should undergo additional evaluation, including imaging.
CHALLENGES TO IMPLEMENTATION: Rest is contrary to usual approach
Some physicians may not agree with the recommendation to encourage rest. Indeed, rest and immobilization are contrary to the usual recommendation for musculoskeletal injuries—to resume activity as soon as possible.
Patients might not like wearing a collar for a variety of personal reasons, such as cosmetic appearance or limitations of motion. On the other hand, some patients may feel that their pain is too severe to be able to participate in PT—which may also be too expensive for, or not readily available to, some patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
To shorten recovery time for adults with acute cervical radiculopathy, recommend either physical therapy (PT) and a home exercise plan or a cervical collar and rest.1 Both are more effective than a wait-and-see strategy.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ. 2009;339:b3883.
Illustrative case
James M, a 43-year-old self-employed mechanic, came to see you 2 weeks ago because of neck pain radiating to his right shoulder, arm, forearm, and dorsum of his hand. You diagnosed acute right-sided cervical radiculopathy and prescribed a nonsteroidal anti-inflammatory drug.
Today he’s back in your office, reporting that he has experienced only minimal transient relief. You reassure him that the pain will subside within a few months, but james wants to know if you can give him something to speed up his recovery and enable him to return to work.
Each year in the United States, approximately 85 out of every 100,000 adults develop cervical radiculopathy2—a neurologic condition characterized by dysfunction of a cervical spinal nerve, the roots of the nerve, or both. In addition to pain in the neck and the arm on the affected side, patients often develop sensory loss, loss of motor function, and/or reflex changes in the affected nerve-root distribution.
Most patients respond to conservative measures
A nonsurgical approach is the preferred first-line treatment strategy for cervical radiculopathy.3 The Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders—an international network of experts in a number of specialties—found no evidence that surgery provides better long-term outcomes than more conservative treatment.3 Approximately 80% to 90% of patients respond to a conservative approach, with improvements in pain, function, and mood in 3 to 6 months.4,5
There are numerous conservative therapies for cervical radiculopathy, including oral analgesics, rest, cervical traction, short-term immobilization with a cervical collar, PT, a short course of oral corticosteroids, and perineural steroid injections.4-6 These therapies may be used singly or in combination. Until now, however, no high-quality RCTs compared the efficacy of various nonsurgical treatment modalities for acute cervical radiculopathy—and their effectiveness is still subject to debate.
STUDY SUMMARY: Initially, both Tx modes beat wait-and-see
The study by Kuijper et al1 is the first RCT to compare the effectiveness of PT, cervical collars, and a wait-and-see strategy in alleviating symptoms of cervical radiculopathy. Enrollees (N=205) were men and women ages 18 to 75 years who were referred by general practitioners in 3 Dutch hospitals. All the participants had a diagnosis of cervical radiculopathy confirmed by a neurologist. In addition, all the cases were of recent onset, with symptoms of <1 month’s duration at the time of enrollment. Patients with clinical signs of cord compression and those who had previously been treated with either PT or a cervical collar for this episode were excluded.
The researchers randomized the participants into 3 groups: PT, cervical collar, or control. All the groups were comparable at baseline.
Those in the PT group received twice weekly therapy for 6 weeks, with a focus on mobilizing and stabilizing the cervical spine. They were also taught to perform home exercises and advised to do the exercises daily.
Patients in the cervical collar group were given a semi-hard, snugly fitted collar and instructed to wear it during the day for 3 weeks—and to rest as much as possible. They were weaned from the collar over the course of another 3 weeks.
Participants in the control group were simply told to follow their normal daily routine as much as possible. All 3 groups were permitted to take oral pain medication as needed.
The primary outcome measures were changes over time in neck and arm pain scores, using 2 validated measurement tools: a 100-mm visual analog scale (VAS) and a 100-point neck disability index (NDI). Both tools were used at 3 weeks, 6 weeks, and 6 months. Secondary outcomes were treatment satisfaction (as measured on a 5-point scale), use of opiates, and working status.
By 6 months, differences virtually disappeared
Both the active and passive interventions reduced arm and neck pain faster than the wait-and-see strategy. At 6 weeks, participants in both the PT and cervical collar groups reported a 31-mm reduction in arm pain (P=.007 and .006, respectively), compared with a 19-mm reduction for those in the control group (P=.006). This is a clinically meaningful difference.
The rate of reduction in neck pain over the first 6 weeks was: PT group, 2.4 mm/week, P=.002; cervical collar group, 2.8 mm/week, P=<.001; and control group, 0.9 mm/week. The rate of reduction in the NDI was 1.4 points per week for the control group vs 2.3 points per week for the cervical collar group (P= .024). The PT group fared no better on the NDI measure than the control group. This may reflect the fact that the index predominantly measured disability caused by neck pain, whereas arm pain scores,—which were highest initially—showed the greatest improvement, the authors note.
At 6 months, pain and disability had almost resolved for all the patients, regardless of their treatment group, and secondary outcomes—treatment satisfaction, analgesic use, and working status—were similar for all 3 groups.
WHAT'S NEW: High-quality RCT supports PT and cervical collar
Some investigators have advocated the short-term use of immobilization with either a cervical collar or a cervical pillow during sleep. Until now, however, there was no conclusive evidence about the benefits of this approach.
One earlier RCT (N=493) compared 5 treatment modalities—traction, positioning, collar, placebo tablets, and heat treatment—and found no significant difference in pain and ability to work.7 That trial was done nearly 15 years ago, however, and the investigators did not use validated outcome scales. Therefore, the trial would not meet current RCT standards.
The study we report on here leaves little doubt that the 2 treatments reviewed—PT and cervical collar—provide more rapid relief than a wait-and-see approach.
CAVEATS: Pain meds still needed, unanswered questions remain
Although the cervical collar and PT groups had less pain at 3 and 6 weeks compared with the controls—and all 3 groups showed equal improvement at study’s end—the researchers found little difference in use of analgesics. Data on adherence to treatment was recorded by patients, so treatment adherence may not be completely accurate.8
Patients without severe arm pain or signs of muscle weakness were not included in this study, so we don’t know whether individuals with less severe cervical radiculopathy would benefit from these treatments. What’s more, this study focused only on new cases of acute cervical radiculopathy, and the findings may not apply to patients with chronic, recurrent, or persistent symptoms.
The apparent contradiction in the finding that both immobilization and PT are beneficial does not have a clear scientific explanation. The researchers hypothesize that immobilizing the neck with a collar reduces foraminal root compression and inflammation; this could explain the larger reduction in arm pain compared with neck pain and neck disability found in this study. The mechanism of pain reduction with PT is unclear, although it is probably related to the restoration of the neck musculature’s strength and range of motion.
Cost is another issue. A cervical collar and rest is at least as effective as PT for recent onset cervical radiculopathy, but the collar costs only about $20—far less than the cost of 12 sessions of therapy.
One final caveat: Any patient with persistent or worsening symptoms should undergo additional evaluation, including imaging.
CHALLENGES TO IMPLEMENTATION: Rest is contrary to usual approach
Some physicians may not agree with the recommendation to encourage rest. Indeed, rest and immobilization are contrary to the usual recommendation for musculoskeletal injuries—to resume activity as soon as possible.
Patients might not like wearing a collar for a variety of personal reasons, such as cosmetic appearance or limitations of motion. On the other hand, some patients may feel that their pain is too severe to be able to participate in PT—which may also be too expensive for, or not readily available to, some patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ 2009;339:b3883.-
2. Radhakrishan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990 Brain 1994;117:325-335.
3. Nordin M, Carragee EJ, Hogg-Johnson S, et al. Assessment of neck pain and its associated disorders: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008;33(suppl 4):S101-S122.
4. Saal JS, Saal JA, Yurth EF. Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine 1996;21:1877-1883.
5. Persson LC, Carlsson CA, Carlsson JY. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar: a prospective, randomized study. Spine 1997;22:751-758.
6. Wolff MW, Levine LA. Cervical radiculopathies: conservative approaches to management. Phys Med Rehabil Clin N Am 2002;13:589-608.
7. Levine MJ, Albert TJ, Smith MD. Cervical radiculopathy: diagnosis and nonoperative management. J Am Acad Orthop Surg. 1996;4:305-316.
8. Wainner RS, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine 2003;28:52-62.
1. Kuijper B, Tans JT, Beelen A, et al. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: randomized trial. BMJ 2009;339:b3883.-
2. Radhakrishan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990 Brain 1994;117:325-335.
3. Nordin M, Carragee EJ, Hogg-Johnson S, et al. Assessment of neck pain and its associated disorders: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008;33(suppl 4):S101-S122.
4. Saal JS, Saal JA, Yurth EF. Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine 1996;21:1877-1883.
5. Persson LC, Carlsson CA, Carlsson JY. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar: a prospective, randomized study. Spine 1997;22:751-758.
6. Wolff MW, Levine LA. Cervical radiculopathies: conservative approaches to management. Phys Med Rehabil Clin N Am 2002;13:589-608.
7. Levine MJ, Albert TJ, Smith MD. Cervical radiculopathy: diagnosis and nonoperative management. J Am Acad Orthop Surg. 1996;4:305-316.
8. Wainner RS, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine 2003;28:52-62.
Copyright © 2010 The Family Physicians Inquiries Network.
All rights reserved.
Initiating antidepressant therapy? Try these 2 drugs first
When you initiate antidepressant therapy for patients who have not been treated for depression previously, select either sertraline or escitalopram. A large meta-analysis found these medications to be superior to other “new-generation” antidepressants.1
Strength of recommendation
A: Meta-analysis of 117 high-quality studies.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
ILLUSTRATIVE CASE
Mrs. D is a 45-year-old patient whom you’ve treated for type 2 diabetes for several years. On her latest visit, she reports a loss of energy and difficulty sleeping and wonders if they could be related to the diabetes. As you explore further and question Mrs. D about these symptoms, she becomes tearful—and tells you she has episodes of sadness and no longer enjoys things the way she used to. Although she has no past history of depression, when you suggest that her symptoms may be an indication of depression, she readily agrees.
You discuss treatment options, including antidepressants and therapy. Mrs. D decides to try medication. But with so many antidepressants on the market, how do you determine which to choose?
Major depression is the fourth leading cause of disease globally, according to the World Health Organization.2 Depression is common in the United States as well, and primary care physicians are often the ones who are diagnosing and treating it. In fact, the US Preventive Services Task Force recently expanded its recommendation that primary care providers screen adults for depression, to include adolescents ages 12 to 18 years.3 When depression is diagnosed, physicians must help patients decide on an initial treatment plan.
All antidepressants are not equal
Options for initial treatment of unipolar major depression include psychotherapy and the use of an antidepressant. For mild and moderate depression, psychotherapy alone is as effective as medication. Combined psychotherapy and antidepressants are more effective than either treatment alone for all degrees of depression.4
The ideal medication for depression would be a drug with a high level of effectiveness and a low side-effect profile; until now, however, there has been little evidence to support 1 antidepressant over another. Previous meta-analyses have concluded that there are no significant differences in either efficacy or acceptability among the various second-generation antidepressants on the market.5,6 Thus, physicians have historically made initial monotherapy treatment decisions based on side effects and cost.7,8 The meta-analysis we report on here tells a different story, providing strong evidence that some antidepressants are more effective and better tolerated than others.
STUDY SUMMARY: Meta-analysis reveals 2 “best” drugs
Cipriani et al1 conducted a systematic review and multiple-treatments meta-analysis of 117 prospective randomized controlled trials (RCTs). Taken together, the RCTs evaluated the comparative efficacy and acceptability of 12 second-generation antidepressants: bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, mirtazapine, paroxetine, reboxetine, sertraline, and venlafaxine. The methodology of this meta-analysis differed from that of traditional meta-analyses by allowing the integration of data from both direct and indirect comparisons. (An indirect comparison is one in which drugs from different trials are assessed by combining the results of their effectiveness and comparing the combined finding with the effectiveness of a drug that all the trials have in common.) Previous studies, based only on direct comparisons, yielded inconsistent results.
The studies included in this meta-analysis were all RCTs in which 1 of these 12 antidepressants was tested against 1, or several, other second-generation antidepressants as monotherapy for the acute treatment phase of unipolar major depression. The authors excluded placebo-controlled trials in order to evaluate efficacy and acceptability of the study medications relative to other commonly used antidepressants. They defined acute treatment as 8 weeks of antidepressant therapy, with a range of 6 to 12 weeks. The primary outcomes studied were response to treatment and dropout rate.
Response to treatment (efficacy) was constructed as a Yes or No variable; a positive response was defined as a reduction of ≥50% in symptom score on either the Hamilton depression rating scale or the Montgomery-Asberg rating scale, or a rating of “improved” or “very much improved” on the clinical global impression at 8 weeks. Efficacy was calculated on an intention-to-treat basis; if data were missing for a participant, that person was classified as a nonresponder.
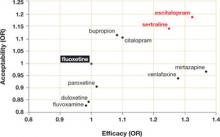
Dropout rate was used to represent acceptability, as the authors believed it to be a more clinically meaningful measure than either side effects or symptom scores. Comparative efficacy and acceptability were analyzed. Fluoxetine—the first of the second-generation antidepressants—was used as the reference medication. The ( FIGURE ) shows the outcomes for 9 of the antidepressants, compared with those of fluoxetine. The other 2 antidepressants, milnacipran and reboxetine, are omitted because they are not available in the United States.
The overall meta-analysis included 25,928 individuals, with 24,595 in the efficacy analysis and 24,693 in the acceptability analysis. Nearly two-thirds (64%) of the participants were women. The mean duration of follow-up was 8.1 weeks, and mean sample size per study was 110. Studies of women with postpartum depression were excluded.
Escitalopram and sertraline stand out. Overall, escitalopram, mirtazapine, sertraline, and venlafaxine were significantly more efficacious than fluoxetine or the other medications. Bupropion, citalopram, escitalopram, and sertraline were better tolerated than the other antidepressants. Escitalopram and sertraline were found to have the best combination of efficacy and acceptability.
Efficacy results. Fifty-nine percent of participants responded to sertraline, vs a 52% response rate for fluoxetine (number needed to treat [NNT]=14). Similarly, 52% of participants responded to escitalopram, compared with 47% of those taking fluoxetine (NNT=20).
Acceptability results. In terms of dropout rate, 28% of participants discontinued fluoxetine, vs 24% of patients taking sertraline. This means that 25 patients would need to be treated with sertraline, rather than fluoxetine, to avoid 1 discontinuation. In the comparison of fluoxetine vs escitalopram, 25% discontinued fluoxetine, compared with 24% who discontinued escitalopram.
The efficacy and acceptability of sertraline and escitalopram compared with other second-generation antidepressant medications show similar trends.
The generic advantage. The investigators recommend sertraline as the best choice for an initial antidepressant because it is available in generic form and is therefore lower in cost. They further recommend that sertraline, instead of fluoxetine or placebo, be the new standard against which other antidepressants are compared.
FIGURE
Sertraline and escitalopram come out on top
Using fluoxetine as the reference medication, the researchers analyzed various second-generation antidepressants. Sertraline and escitalopram had the best combination of efficacy and acceptability.
OR, odds ratio.
Source: Cipriani A et al. Lancet. 2009.1
WHAT’S NEW?: Antidepressant choice is evidence-based
We now have solid evidence for choosing sertraline or escitalopram as the first medication to use when treating a patient with newly diagnosed depression. This represents a practice change because antidepressants that are less effective and less acceptable have been chosen more frequently than either of these medications. That conclusion is based on our analysis of the National Ambulatory Medical Care Survey database for outpatient and ambulatory clinic visits in 2005-2006 (the most recent data available). We conducted this analysis to determine which of the second-generation antidepressants were prescribed most for initial monotherapy of major depression.
Our finding: An estimated 4 million patients ages 18 years and older diagnosed with depression in the course of the study year received new prescriptions for a single antidepressant. Six medications accounted for 90% of the prescriptions, in the following order:
- fluoxetine (Prozac)
- duloxetine (Cymbalta)
- escitalopram (Lexapro)
- paroxetine (Paxil)
- venlafaxine (Effexor)
- sertraline (Zoloft).
Sertraline and escitalopram, the drugs shown to be most effective and acceptable in the Cipriani meta-analysis, accounted for 11.8% and 14.5% of the prescriptions, respectively.
CAVEATS: Meta-analysis looked only at acute treatment phase
The results of this study are limited to initial therapy as measured at 8 weeks. Little long-term outcome data are available; response to initial therapy may not be a predictor of full remission or long-term success. Current guidelines suggest maintenance of the initial successful therapy, often with increasing intervals between visits, to prevent relapse.9
This study does not add new insight into long-term response rates. Nor does it deal with choice of a replacement or second antidepressant for nonresponders or those who cannot tolerate the initial drug.
What’s more, the study covers drug treatment alone, which may not be the best initial treatment for depression. Psychotherapy, in the form of cognitive behavioral therapy or interpersonal therapy, when available, is equally effective, has fewer potential physiologic side effects, and may produce longer-lasting results.10,11
Little is known about study design
The authors of this study had access only to limited information about inclusion criteria and the composition of initial study populations or settings. There is a difference between a trial designed to evaluate the “efficacy” of an intervention (“the beneficial and harmful effects of an intervention under controlled circumstances”) and the “effectiveness” of an intervention (the “beneficial and harmful effects of the intervention under usual circumstances”).12 It is not clear which of the 117 studies were efficacy studies and which were effectiveness studies. This may limit the overall generalizability of the study results to a primary care population.
Studies included in this meta-analysis were selected exclusively from published literature. There is some evidence that there is a bias toward the publication of studies with positive results, which may have the effect of overstating the effectiveness of a given antidepressant.13 However, we have no reason to believe that this bias would favor any particular drug.
Most of the included studies were sponsored by drug companies. Notably, pharmaceutical companies have the option of continuing to conduct trials of medications until a study results in a positive finding for their medication, with no penalty for the suppression of equivocal or negative results (negative publication bias). Under current FDA guidelines, there is little transparency to the consumer as to how many trials have been undertaken and the direction of the results, published or unpublished.14
We doubt that either publication bias or the design and sponsorship of the studies included in this meta-analysis present significant threats to the validity of these findings over other sources upon which guidelines rely, given that these issues are common to much of the research on pharmacologic therapy. We also doubt that the compensation of the authors by pharmaceutical companies would bias the outcome of the study in this instance. One of the authors (TAF) received compensation from Pfizer, the maker of Zoloft, which is also available as generic sertraline. None of the authors received compensation from Forest Pharmaceuticals, the makers of Lexapro (escitalopram).
CHALLENGES TO IMPLEMENTATION: No major barriers are anticipated
Both sertraline and escitalopram are covered by most health insurers. As noted above, sertraline is available in generic formulation, and is therefore much less expensive than escitalopram. In a check of online drug prices, we found a prescription for a 3-month supply of Lexapro (10 mg) to cost about $250; a 3-month supply of generic sertraline (100 mg) from the same sources would cost approximately $35 (www.pharmcychecker.com). Both Pfizer, the maker of Zoloft, and Forest Pharmaceuticals, the maker of Lexapro, have patient assistance programs to make these medications available to low-income, uninsured patients.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR02499 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
1. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
2. Murray CJ, Lopez AD. Global Burden of Disease. Cambridge, MA: Harvard University Press; 1996.
3. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the U.S. Preventive Services Task Force. Pediatrics. 2009;123:e716-e735.
4. Timonen M, Liukkonen T. Management of depression in adults. BMJ. 2008;336:435-439.
5. Gartlehner G, Hansen RA, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression. Comparative Effectiveness Review No. 7. (Prepared by RTI International-University of North Carolina Evidence Based Practice Center under Contract No. 290-02-0016.) Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed May 18, 2009.
6. Hansen RA, Gartlehner G, Lohr KN, et al. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415-426.
7. Adams SM, Miller KE, Zylstra RG. Pharmacologic management of adult depression. Am Fam Physician. 2008;77:785-792.
8. Qaseem A, Snow V, Denberg TD, et al. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
9. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409-416.
10. deMello MF, de Jesus MJ, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
11. APA Practice Guidelines. Practice guideline for the treatment of patients with major depressive disorder, second edition. Available at: http://www.psychiatryonline.com/content.aspx?aID=48727. Accessed June 16, 2009.
12. Sackett D. An introduction to performing therapeutic trials. In: Haynes RB, Sackett DL, et al, eds. Clinical Epidemiology: How to Do Clinical Practice Research. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
13. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
14. Mathew SJ, Charney DS. Publication bias and the efficacy of antidepressants. Am J Psychiatry. 2009;166:140-145.
PURLs methodology This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
When you initiate antidepressant therapy for patients who have not been treated for depression previously, select either sertraline or escitalopram. A large meta-analysis found these medications to be superior to other “new-generation” antidepressants.1
Strength of recommendation
A: Meta-analysis of 117 high-quality studies.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
ILLUSTRATIVE CASE
Mrs. D is a 45-year-old patient whom you’ve treated for type 2 diabetes for several years. On her latest visit, she reports a loss of energy and difficulty sleeping and wonders if they could be related to the diabetes. As you explore further and question Mrs. D about these symptoms, she becomes tearful—and tells you she has episodes of sadness and no longer enjoys things the way she used to. Although she has no past history of depression, when you suggest that her symptoms may be an indication of depression, she readily agrees.
You discuss treatment options, including antidepressants and therapy. Mrs. D decides to try medication. But with so many antidepressants on the market, how do you determine which to choose?
Major depression is the fourth leading cause of disease globally, according to the World Health Organization.2 Depression is common in the United States as well, and primary care physicians are often the ones who are diagnosing and treating it. In fact, the US Preventive Services Task Force recently expanded its recommendation that primary care providers screen adults for depression, to include adolescents ages 12 to 18 years.3 When depression is diagnosed, physicians must help patients decide on an initial treatment plan.
All antidepressants are not equal
Options for initial treatment of unipolar major depression include psychotherapy and the use of an antidepressant. For mild and moderate depression, psychotherapy alone is as effective as medication. Combined psychotherapy and antidepressants are more effective than either treatment alone for all degrees of depression.4
The ideal medication for depression would be a drug with a high level of effectiveness and a low side-effect profile; until now, however, there has been little evidence to support 1 antidepressant over another. Previous meta-analyses have concluded that there are no significant differences in either efficacy or acceptability among the various second-generation antidepressants on the market.5,6 Thus, physicians have historically made initial monotherapy treatment decisions based on side effects and cost.7,8 The meta-analysis we report on here tells a different story, providing strong evidence that some antidepressants are more effective and better tolerated than others.
STUDY SUMMARY: Meta-analysis reveals 2 “best” drugs
Cipriani et al1 conducted a systematic review and multiple-treatments meta-analysis of 117 prospective randomized controlled trials (RCTs). Taken together, the RCTs evaluated the comparative efficacy and acceptability of 12 second-generation antidepressants: bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, mirtazapine, paroxetine, reboxetine, sertraline, and venlafaxine. The methodology of this meta-analysis differed from that of traditional meta-analyses by allowing the integration of data from both direct and indirect comparisons. (An indirect comparison is one in which drugs from different trials are assessed by combining the results of their effectiveness and comparing the combined finding with the effectiveness of a drug that all the trials have in common.) Previous studies, based only on direct comparisons, yielded inconsistent results.
The studies included in this meta-analysis were all RCTs in which 1 of these 12 antidepressants was tested against 1, or several, other second-generation antidepressants as monotherapy for the acute treatment phase of unipolar major depression. The authors excluded placebo-controlled trials in order to evaluate efficacy and acceptability of the study medications relative to other commonly used antidepressants. They defined acute treatment as 8 weeks of antidepressant therapy, with a range of 6 to 12 weeks. The primary outcomes studied were response to treatment and dropout rate.
Response to treatment (efficacy) was constructed as a Yes or No variable; a positive response was defined as a reduction of ≥50% in symptom score on either the Hamilton depression rating scale or the Montgomery-Asberg rating scale, or a rating of “improved” or “very much improved” on the clinical global impression at 8 weeks. Efficacy was calculated on an intention-to-treat basis; if data were missing for a participant, that person was classified as a nonresponder.
Dropout rate was used to represent acceptability, as the authors believed it to be a more clinically meaningful measure than either side effects or symptom scores. Comparative efficacy and acceptability were analyzed. Fluoxetine—the first of the second-generation antidepressants—was used as the reference medication. The ( FIGURE ) shows the outcomes for 9 of the antidepressants, compared with those of fluoxetine. The other 2 antidepressants, milnacipran and reboxetine, are omitted because they are not available in the United States.
The overall meta-analysis included 25,928 individuals, with 24,595 in the efficacy analysis and 24,693 in the acceptability analysis. Nearly two-thirds (64%) of the participants were women. The mean duration of follow-up was 8.1 weeks, and mean sample size per study was 110. Studies of women with postpartum depression were excluded.
Escitalopram and sertraline stand out. Overall, escitalopram, mirtazapine, sertraline, and venlafaxine were significantly more efficacious than fluoxetine or the other medications. Bupropion, citalopram, escitalopram, and sertraline were better tolerated than the other antidepressants. Escitalopram and sertraline were found to have the best combination of efficacy and acceptability.
Efficacy results. Fifty-nine percent of participants responded to sertraline, vs a 52% response rate for fluoxetine (number needed to treat [NNT]=14). Similarly, 52% of participants responded to escitalopram, compared with 47% of those taking fluoxetine (NNT=20).
Acceptability results. In terms of dropout rate, 28% of participants discontinued fluoxetine, vs 24% of patients taking sertraline. This means that 25 patients would need to be treated with sertraline, rather than fluoxetine, to avoid 1 discontinuation. In the comparison of fluoxetine vs escitalopram, 25% discontinued fluoxetine, compared with 24% who discontinued escitalopram.
The efficacy and acceptability of sertraline and escitalopram compared with other second-generation antidepressant medications show similar trends.
The generic advantage. The investigators recommend sertraline as the best choice for an initial antidepressant because it is available in generic form and is therefore lower in cost. They further recommend that sertraline, instead of fluoxetine or placebo, be the new standard against which other antidepressants are compared.
FIGURE
Sertraline and escitalopram come out on top
Using fluoxetine as the reference medication, the researchers analyzed various second-generation antidepressants. Sertraline and escitalopram had the best combination of efficacy and acceptability.
OR, odds ratio.
Source: Cipriani A et al. Lancet. 2009.1
WHAT’S NEW?: Antidepressant choice is evidence-based
We now have solid evidence for choosing sertraline or escitalopram as the first medication to use when treating a patient with newly diagnosed depression. This represents a practice change because antidepressants that are less effective and less acceptable have been chosen more frequently than either of these medications. That conclusion is based on our analysis of the National Ambulatory Medical Care Survey database for outpatient and ambulatory clinic visits in 2005-2006 (the most recent data available). We conducted this analysis to determine which of the second-generation antidepressants were prescribed most for initial monotherapy of major depression.
Our finding: An estimated 4 million patients ages 18 years and older diagnosed with depression in the course of the study year received new prescriptions for a single antidepressant. Six medications accounted for 90% of the prescriptions, in the following order:
- fluoxetine (Prozac)
- duloxetine (Cymbalta)
- escitalopram (Lexapro)
- paroxetine (Paxil)
- venlafaxine (Effexor)
- sertraline (Zoloft).
Sertraline and escitalopram, the drugs shown to be most effective and acceptable in the Cipriani meta-analysis, accounted for 11.8% and 14.5% of the prescriptions, respectively.
CAVEATS: Meta-analysis looked only at acute treatment phase
The results of this study are limited to initial therapy as measured at 8 weeks. Little long-term outcome data are available; response to initial therapy may not be a predictor of full remission or long-term success. Current guidelines suggest maintenance of the initial successful therapy, often with increasing intervals between visits, to prevent relapse.9
This study does not add new insight into long-term response rates. Nor does it deal with choice of a replacement or second antidepressant for nonresponders or those who cannot tolerate the initial drug.
What’s more, the study covers drug treatment alone, which may not be the best initial treatment for depression. Psychotherapy, in the form of cognitive behavioral therapy or interpersonal therapy, when available, is equally effective, has fewer potential physiologic side effects, and may produce longer-lasting results.10,11
Little is known about study design
The authors of this study had access only to limited information about inclusion criteria and the composition of initial study populations or settings. There is a difference between a trial designed to evaluate the “efficacy” of an intervention (“the beneficial and harmful effects of an intervention under controlled circumstances”) and the “effectiveness” of an intervention (the “beneficial and harmful effects of the intervention under usual circumstances”).12 It is not clear which of the 117 studies were efficacy studies and which were effectiveness studies. This may limit the overall generalizability of the study results to a primary care population.
Studies included in this meta-analysis were selected exclusively from published literature. There is some evidence that there is a bias toward the publication of studies with positive results, which may have the effect of overstating the effectiveness of a given antidepressant.13 However, we have no reason to believe that this bias would favor any particular drug.
Most of the included studies were sponsored by drug companies. Notably, pharmaceutical companies have the option of continuing to conduct trials of medications until a study results in a positive finding for their medication, with no penalty for the suppression of equivocal or negative results (negative publication bias). Under current FDA guidelines, there is little transparency to the consumer as to how many trials have been undertaken and the direction of the results, published or unpublished.14
We doubt that either publication bias or the design and sponsorship of the studies included in this meta-analysis present significant threats to the validity of these findings over other sources upon which guidelines rely, given that these issues are common to much of the research on pharmacologic therapy. We also doubt that the compensation of the authors by pharmaceutical companies would bias the outcome of the study in this instance. One of the authors (TAF) received compensation from Pfizer, the maker of Zoloft, which is also available as generic sertraline. None of the authors received compensation from Forest Pharmaceuticals, the makers of Lexapro (escitalopram).
CHALLENGES TO IMPLEMENTATION: No major barriers are anticipated
Both sertraline and escitalopram are covered by most health insurers. As noted above, sertraline is available in generic formulation, and is therefore much less expensive than escitalopram. In a check of online drug prices, we found a prescription for a 3-month supply of Lexapro (10 mg) to cost about $250; a 3-month supply of generic sertraline (100 mg) from the same sources would cost approximately $35 (www.pharmcychecker.com). Both Pfizer, the maker of Zoloft, and Forest Pharmaceuticals, the maker of Lexapro, have patient assistance programs to make these medications available to low-income, uninsured patients.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR02499 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
When you initiate antidepressant therapy for patients who have not been treated for depression previously, select either sertraline or escitalopram. A large meta-analysis found these medications to be superior to other “new-generation” antidepressants.1
Strength of recommendation
A: Meta-analysis of 117 high-quality studies.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
ILLUSTRATIVE CASE
Mrs. D is a 45-year-old patient whom you’ve treated for type 2 diabetes for several years. On her latest visit, she reports a loss of energy and difficulty sleeping and wonders if they could be related to the diabetes. As you explore further and question Mrs. D about these symptoms, she becomes tearful—and tells you she has episodes of sadness and no longer enjoys things the way she used to. Although she has no past history of depression, when you suggest that her symptoms may be an indication of depression, she readily agrees.
You discuss treatment options, including antidepressants and therapy. Mrs. D decides to try medication. But with so many antidepressants on the market, how do you determine which to choose?
Major depression is the fourth leading cause of disease globally, according to the World Health Organization.2 Depression is common in the United States as well, and primary care physicians are often the ones who are diagnosing and treating it. In fact, the US Preventive Services Task Force recently expanded its recommendation that primary care providers screen adults for depression, to include adolescents ages 12 to 18 years.3 When depression is diagnosed, physicians must help patients decide on an initial treatment plan.
All antidepressants are not equal
Options for initial treatment of unipolar major depression include psychotherapy and the use of an antidepressant. For mild and moderate depression, psychotherapy alone is as effective as medication. Combined psychotherapy and antidepressants are more effective than either treatment alone for all degrees of depression.4
The ideal medication for depression would be a drug with a high level of effectiveness and a low side-effect profile; until now, however, there has been little evidence to support 1 antidepressant over another. Previous meta-analyses have concluded that there are no significant differences in either efficacy or acceptability among the various second-generation antidepressants on the market.5,6 Thus, physicians have historically made initial monotherapy treatment decisions based on side effects and cost.7,8 The meta-analysis we report on here tells a different story, providing strong evidence that some antidepressants are more effective and better tolerated than others.
STUDY SUMMARY: Meta-analysis reveals 2 “best” drugs
Cipriani et al1 conducted a systematic review and multiple-treatments meta-analysis of 117 prospective randomized controlled trials (RCTs). Taken together, the RCTs evaluated the comparative efficacy and acceptability of 12 second-generation antidepressants: bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, mirtazapine, paroxetine, reboxetine, sertraline, and venlafaxine. The methodology of this meta-analysis differed from that of traditional meta-analyses by allowing the integration of data from both direct and indirect comparisons. (An indirect comparison is one in which drugs from different trials are assessed by combining the results of their effectiveness and comparing the combined finding with the effectiveness of a drug that all the trials have in common.) Previous studies, based only on direct comparisons, yielded inconsistent results.
The studies included in this meta-analysis were all RCTs in which 1 of these 12 antidepressants was tested against 1, or several, other second-generation antidepressants as monotherapy for the acute treatment phase of unipolar major depression. The authors excluded placebo-controlled trials in order to evaluate efficacy and acceptability of the study medications relative to other commonly used antidepressants. They defined acute treatment as 8 weeks of antidepressant therapy, with a range of 6 to 12 weeks. The primary outcomes studied were response to treatment and dropout rate.
Response to treatment (efficacy) was constructed as a Yes or No variable; a positive response was defined as a reduction of ≥50% in symptom score on either the Hamilton depression rating scale or the Montgomery-Asberg rating scale, or a rating of “improved” or “very much improved” on the clinical global impression at 8 weeks. Efficacy was calculated on an intention-to-treat basis; if data were missing for a participant, that person was classified as a nonresponder.
Dropout rate was used to represent acceptability, as the authors believed it to be a more clinically meaningful measure than either side effects or symptom scores. Comparative efficacy and acceptability were analyzed. Fluoxetine—the first of the second-generation antidepressants—was used as the reference medication. The ( FIGURE ) shows the outcomes for 9 of the antidepressants, compared with those of fluoxetine. The other 2 antidepressants, milnacipran and reboxetine, are omitted because they are not available in the United States.
The overall meta-analysis included 25,928 individuals, with 24,595 in the efficacy analysis and 24,693 in the acceptability analysis. Nearly two-thirds (64%) of the participants were women. The mean duration of follow-up was 8.1 weeks, and mean sample size per study was 110. Studies of women with postpartum depression were excluded.
Escitalopram and sertraline stand out. Overall, escitalopram, mirtazapine, sertraline, and venlafaxine were significantly more efficacious than fluoxetine or the other medications. Bupropion, citalopram, escitalopram, and sertraline were better tolerated than the other antidepressants. Escitalopram and sertraline were found to have the best combination of efficacy and acceptability.
Efficacy results. Fifty-nine percent of participants responded to sertraline, vs a 52% response rate for fluoxetine (number needed to treat [NNT]=14). Similarly, 52% of participants responded to escitalopram, compared with 47% of those taking fluoxetine (NNT=20).
Acceptability results. In terms of dropout rate, 28% of participants discontinued fluoxetine, vs 24% of patients taking sertraline. This means that 25 patients would need to be treated with sertraline, rather than fluoxetine, to avoid 1 discontinuation. In the comparison of fluoxetine vs escitalopram, 25% discontinued fluoxetine, compared with 24% who discontinued escitalopram.
The efficacy and acceptability of sertraline and escitalopram compared with other second-generation antidepressant medications show similar trends.
The generic advantage. The investigators recommend sertraline as the best choice for an initial antidepressant because it is available in generic form and is therefore lower in cost. They further recommend that sertraline, instead of fluoxetine or placebo, be the new standard against which other antidepressants are compared.
FIGURE
Sertraline and escitalopram come out on top
Using fluoxetine as the reference medication, the researchers analyzed various second-generation antidepressants. Sertraline and escitalopram had the best combination of efficacy and acceptability.
OR, odds ratio.
Source: Cipriani A et al. Lancet. 2009.1
WHAT’S NEW?: Antidepressant choice is evidence-based
We now have solid evidence for choosing sertraline or escitalopram as the first medication to use when treating a patient with newly diagnosed depression. This represents a practice change because antidepressants that are less effective and less acceptable have been chosen more frequently than either of these medications. That conclusion is based on our analysis of the National Ambulatory Medical Care Survey database for outpatient and ambulatory clinic visits in 2005-2006 (the most recent data available). We conducted this analysis to determine which of the second-generation antidepressants were prescribed most for initial monotherapy of major depression.
Our finding: An estimated 4 million patients ages 18 years and older diagnosed with depression in the course of the study year received new prescriptions for a single antidepressant. Six medications accounted for 90% of the prescriptions, in the following order:
- fluoxetine (Prozac)
- duloxetine (Cymbalta)
- escitalopram (Lexapro)
- paroxetine (Paxil)
- venlafaxine (Effexor)
- sertraline (Zoloft).
Sertraline and escitalopram, the drugs shown to be most effective and acceptable in the Cipriani meta-analysis, accounted for 11.8% and 14.5% of the prescriptions, respectively.
CAVEATS: Meta-analysis looked only at acute treatment phase
The results of this study are limited to initial therapy as measured at 8 weeks. Little long-term outcome data are available; response to initial therapy may not be a predictor of full remission or long-term success. Current guidelines suggest maintenance of the initial successful therapy, often with increasing intervals between visits, to prevent relapse.9
This study does not add new insight into long-term response rates. Nor does it deal with choice of a replacement or second antidepressant for nonresponders or those who cannot tolerate the initial drug.
What’s more, the study covers drug treatment alone, which may not be the best initial treatment for depression. Psychotherapy, in the form of cognitive behavioral therapy or interpersonal therapy, when available, is equally effective, has fewer potential physiologic side effects, and may produce longer-lasting results.10,11
Little is known about study design
The authors of this study had access only to limited information about inclusion criteria and the composition of initial study populations or settings. There is a difference between a trial designed to evaluate the “efficacy” of an intervention (“the beneficial and harmful effects of an intervention under controlled circumstances”) and the “effectiveness” of an intervention (the “beneficial and harmful effects of the intervention under usual circumstances”).12 It is not clear which of the 117 studies were efficacy studies and which were effectiveness studies. This may limit the overall generalizability of the study results to a primary care population.
Studies included in this meta-analysis were selected exclusively from published literature. There is some evidence that there is a bias toward the publication of studies with positive results, which may have the effect of overstating the effectiveness of a given antidepressant.13 However, we have no reason to believe that this bias would favor any particular drug.
Most of the included studies were sponsored by drug companies. Notably, pharmaceutical companies have the option of continuing to conduct trials of medications until a study results in a positive finding for their medication, with no penalty for the suppression of equivocal or negative results (negative publication bias). Under current FDA guidelines, there is little transparency to the consumer as to how many trials have been undertaken and the direction of the results, published or unpublished.14
We doubt that either publication bias or the design and sponsorship of the studies included in this meta-analysis present significant threats to the validity of these findings over other sources upon which guidelines rely, given that these issues are common to much of the research on pharmacologic therapy. We also doubt that the compensation of the authors by pharmaceutical companies would bias the outcome of the study in this instance. One of the authors (TAF) received compensation from Pfizer, the maker of Zoloft, which is also available as generic sertraline. None of the authors received compensation from Forest Pharmaceuticals, the makers of Lexapro (escitalopram).
CHALLENGES TO IMPLEMENTATION: No major barriers are anticipated
Both sertraline and escitalopram are covered by most health insurers. As noted above, sertraline is available in generic formulation, and is therefore much less expensive than escitalopram. In a check of online drug prices, we found a prescription for a 3-month supply of Lexapro (10 mg) to cost about $250; a 3-month supply of generic sertraline (100 mg) from the same sources would cost approximately $35 (www.pharmcychecker.com). Both Pfizer, the maker of Zoloft, and Forest Pharmaceuticals, the maker of Lexapro, have patient assistance programs to make these medications available to low-income, uninsured patients.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR02499 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
1. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
2. Murray CJ, Lopez AD. Global Burden of Disease. Cambridge, MA: Harvard University Press; 1996.
3. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the U.S. Preventive Services Task Force. Pediatrics. 2009;123:e716-e735.
4. Timonen M, Liukkonen T. Management of depression in adults. BMJ. 2008;336:435-439.
5. Gartlehner G, Hansen RA, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression. Comparative Effectiveness Review No. 7. (Prepared by RTI International-University of North Carolina Evidence Based Practice Center under Contract No. 290-02-0016.) Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed May 18, 2009.
6. Hansen RA, Gartlehner G, Lohr KN, et al. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415-426.
7. Adams SM, Miller KE, Zylstra RG. Pharmacologic management of adult depression. Am Fam Physician. 2008;77:785-792.
8. Qaseem A, Snow V, Denberg TD, et al. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
9. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409-416.
10. deMello MF, de Jesus MJ, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
11. APA Practice Guidelines. Practice guideline for the treatment of patients with major depressive disorder, second edition. Available at: http://www.psychiatryonline.com/content.aspx?aID=48727. Accessed June 16, 2009.
12. Sackett D. An introduction to performing therapeutic trials. In: Haynes RB, Sackett DL, et al, eds. Clinical Epidemiology: How to Do Clinical Practice Research. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
13. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
14. Mathew SJ, Charney DS. Publication bias and the efficacy of antidepressants. Am J Psychiatry. 2009;166:140-145.
PURLs methodology This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746-758.
2. Murray CJ, Lopez AD. Global Burden of Disease. Cambridge, MA: Harvard University Press; 1996.
3. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the U.S. Preventive Services Task Force. Pediatrics. 2009;123:e716-e735.
4. Timonen M, Liukkonen T. Management of depression in adults. BMJ. 2008;336:435-439.
5. Gartlehner G, Hansen RA, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression. Comparative Effectiveness Review No. 7. (Prepared by RTI International-University of North Carolina Evidence Based Practice Center under Contract No. 290-02-0016.) Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed May 18, 2009.
6. Hansen RA, Gartlehner G, Lohr KN, et al. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415-426.
7. Adams SM, Miller KE, Zylstra RG. Pharmacologic management of adult depression. Am Fam Physician. 2008;77:785-792.
8. Qaseem A, Snow V, Denberg TD, et al. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
9. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409-416.
10. deMello MF, de Jesus MJ, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
11. APA Practice Guidelines. Practice guideline for the treatment of patients with major depressive disorder, second edition. Available at: http://www.psychiatryonline.com/content.aspx?aID=48727. Accessed June 16, 2009.
12. Sackett D. An introduction to performing therapeutic trials. In: Haynes RB, Sackett DL, et al, eds. Clinical Epidemiology: How to Do Clinical Practice Research. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
13. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252-260.
14. Mathew SJ, Charney DS. Publication bias and the efficacy of antidepressants. Am J Psychiatry. 2009;166:140-145.
PURLs methodology This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Copyright © 2009 The Family Physicians Inquiries Network.
All rights reserved.
This antiemetic may help kids skip that trip to the hospital
Give oral ondansetron to children with acute gastroenteritis and moderate dehydration who are unable to tolerate oral rehydration to reduce the vomiting and avoid the need for intravenous (IV) hydration or hospitalization.1
Strength of recommendation
A: Meta-analysis of 6 high-quality studies
DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
ILLUSTRATIVE CASE
Sarah, a 2-year-old who has been vomiting and had diarrhea for the past 2 days, is brought to your office by her parents. They tell you she’s unable to tolerate oral fluids, and vomited twice after being given small amounts of juice and soup earlier in the day. Sarah has decreased urine output, but she is not febrile and has no blood in her stools. On examination, you find mild tachycardia, dry mucous membranes, delayed capillary refill, and normal mental status.
You try giving Sarah an oral electrolyte solution, but she vomits immediately. Her parents are reluctant to take her to the emergency department for intravenous (IV) hydration, and ask if you can provide a safe and effective alternative.
Each year in the United States, pediatric gastroenteritis and dehydration are responsible for approximately 1.5 million outpatient visits2 and 150,000 to 170,000 hospital admissions.3 Oral hydration, recommended by pediatric practice guidelines2,4 and the World Health Organization,5 is safe and generally effective. But, as in Sarah’s case, emesis frequently interferes, leading to hospital admission for IV hydration.
An antiemetic with fewer adverse effects
Older antiemetic medications, such as promethazine, prochlorperazine, and metoclopramide, can cause sedation and extrapyramidal reactions. Ondansetron, a selective 5-hydroxytryptamine (5-HT3) receptor antagonist that has been used to control postoperative and chemotherapy-associated nausea and vomiting in children and adults, does not cause either problem. In recent studies of ondansetron’s effectiveness in treating children with gastroenteritis, increased diarrhea, lasting up to 48 hours after administration, was the only adverse event.1
Two earlier systematic reviews—a meta-analysis by Szajewska et al6 and a Cochrane review7—found clinical benefits of ondansetron for vomiting associated with acute gastroenteritis. But both concluded that the evidence was insufficient to recommend routine use of this drug. The meta-analysis that we review below included additional studies, and the researchers reached a different conclusion.
STUDY SUMMARY: Antiemetic decreases vomiting, hospitalization
DeCamp et al conducted a systematic review and meta-analysis of 11 prospective controlled trials that evaluated antiemetic use in children with vomiting from acute gastroenteritis.1 Six of the 11 trials focused on ondansetron;8-13 these 6 were the most recently published and of the highest quality. (The researchers found the remaining 5 trials to be of low methodological quality, with small sample sizes and inconsistent results, and concluded that the antiemetics they assessed should not be used for outpatients with gastroenteritis.) Their meta-analysis of these 6 trials is the focus of this PURL.
The ondansetron studies included a total of 745 children with vomiting and a clinical diagnosis of gastroenteritis. In 5 of the trials, patients received only 1 dose of ondansetron;8-10,12,13 in the sixth, families received additional doses of ondansetron to use at home.11 In 3 trials, patients were given oral ondansetron—a tablet placed on the tongue that dissolves in minutes. The remaining 3 used an IV formulation.8,10,13 Five trials were conducted in emergency departments (EDs),9-13 and 1 in an inpatient setting.8
Big reductions. Children who received ondansetron had significantly less vomiting (16.9% vs 37.8%) and IV fluid administration (13.9% vs 33.9%), and fewer hospital admissions (7.5% vs 14.6%) compared with patients who were given a placebo (TABLE). Diarrhea, the only adverse event to be systematically evaluated, was assessed in all but 1 of the trials.8-12 In 3 of the 5 that reported on this side effect, patients who received ondansetron had an increase in diarrhea for up to 48 hours.8,11,12
TABLE
Ondansetron reduces vomiting, hospitalization, and IV fluid use
| TOTAL NUMBER OF PATIENTS (N=745) | ONDANSETRON | PLACEBO | RR (95% CI) | NNT (95% CI) |
|---|---|---|---|---|
| Continued vomiting (n=659) | 16.9% | 37.8% | 0.45 (0.33-0.62) | 5 (4-7) |
| IV fluid administration (n=489) | 13.9% | 33.9% | 0.41 (0.28-0.62) | 5 (4-8) |
| Hospital admission (n=662) | 7.5% | 14.6% | 0.52 (0.27-0.95) | 14 (9-44) |
| CI, confidence interval; IV, intravenous; NNT, number needed to treat; RR, relative risk. | ||||
| Source: DeCamp LS, et al. Arch Pediatr Adolesc Med.1 | ||||
WHAT’S NEW: Support for a strategy increasingly used in EDs
Physicians are just beginning to adopt the use of ondansetron as a strategy for avoiding IV hydration and hospitalization for children with vomiting associated with minor gastrointestinal illness. As an adjunct to our report on this meta-analysis, we analyzed the use of the antiemetic in children between the ages of 1 and 10 years in emergency visits reported to the National Ambulatory Medical Care Survey database from 2002 to 2006. Among an estimate of more than 3 million pediatric visits to EDs for acute gastroenteritis in each of these years, in 2002 only 0.53% were treated with ondansetron. By 2006, that percentage had risen to 6.43%.
A similar analysis of both ED and outpatient visits to academic medical centers and teaching hospitals from 2005 through 2008 (estimated using data through October 2008), derived from the University Health System Consortium Clinical Database, showed a similar trend. In 2005, only 0.5% of children presenting to EDs and 0.5% of those seeking outpatient care for acute gastritis received ondansetron. By 2008, the numbers had grown to an estimated 3.43% and 3.60%, respectively.
Given the positive results of the DeCamp study and the fact that oral ondansetron is now available in a generic formulation, we expect the use of this antiemetic to increase in both outpatient and emergency settings. We think quite a few IV lines and hospitalizations could be avoided with the use of this antiemetic, not to mention the symptomatic relief for children.
CAVEATS: Studies didn’t look at milder cases, primary care
None of the studies of oral ondansetron for acute gastroenteritis involved outpatient settings, and all 6 of the trials featured children who were moderately ill. It has not yet been determined whether the benefits seen in the ED will apply to an ambulatory population in which many potential candidates for ondansetron have milder gastroenteritis. Nor is it clear whether oral ondansetron would complement oral rehydration in primary care practices. More detailed evaluation of the reduction of vomiting at home over the course of the illness would help to answer these questions.
Nonetheless, ondansetron appears to be safe. Increased diarrhea, the only documented side effect, resolved after 48 hours, and did not appear to result in higher health care utilization.
Don’t prescribe over the phone. It is important to note that all the ondansetron trials included an evaluation of each patient to consider other etiologies, such as central nervous system disorders or toxic exposures, prior to treatment. Physicians are cautioned not to prescribe antiemetics over the telephone—or without first ruling out more serious illnesses in which vomiting is part of the presentation.
Studies were funded by pharma. The primary studies of ondansetron were funded by GlaxoSmithKline, the pharmaceutical company that manufactures the drug under the trade name Zofran. The authors of the meta-analysis reviewed the Clinical Trials Registry and the reference lists of the articles and contacted other experts to find any unreported trials, but found no evidence of negative publication bias. Therefore, we have confidence in these findings. Ideally, additional studies will be conducted without drug company support, in an outpatient setting, to clarify the use of ondansetron as an adjunct to oral rehydration.
CHALLENGES TO IMPLEMENTATION: No major barriers
Cost should not be a barrier to the use of oral ondansetron. The generic formulation sells for $10 to $20 per tablet, and is covered by most health insurers. However, treatment of children with acute gastroenteritis and moderate dehydration in the office setting would likely require a period of observation for tolerance of oral rehydration before and after administration of ondansetron. This may be impractical in some busy clinics.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
3. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97:424-435.
5. World Health Organization. Clinical management of acute diarrhoea. WHO/Unicef joint statement. Available at http://www.who.int/child_adolescent_health/documents/who_fch_cah_04_7/en/index.html. Accessed January 15, 2009.
6. Szajewska H, Gieruszczak-Bialek D, Dylag M. Meta-analysis: ondansetron for vomiting in acute gastroenteritis in children. Aliment Pharmacol Ther. 2007;25:393-400.
7. Alhashimi D, Alhashimi H, Fedorowicz Z. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2006;(4):CD005506.-
8. Cubeddu LX, Trujillo LM, Talmaciu I, et al. Antiemetic activity of ondansetron in acute gastroenteritis. Aliment Pharmacol Ther. 1997;11:185-191.
9. Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52:22-29.
10. Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis; a randomized, controlled trial. Pediatrics. 2002;109:e62.-Available at: http://pediatrics.aappublications.org/cgi/reprint/109/4/e62. Accessed January 12, 2009.
11. Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med. 2002;39:397-403.
12. Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354:1698-1705.
13. Stork CM, Brown KM, Reilly TH, et al. Emergency department treatment of viral gastritis using intravenous ondansetron or dexamethasone in children. Acad Emerg Med. 2006;13:1027-1033.
Give oral ondansetron to children with acute gastroenteritis and moderate dehydration who are unable to tolerate oral rehydration to reduce the vomiting and avoid the need for intravenous (IV) hydration or hospitalization.1
Strength of recommendation
A: Meta-analysis of 6 high-quality studies
DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
ILLUSTRATIVE CASE
Sarah, a 2-year-old who has been vomiting and had diarrhea for the past 2 days, is brought to your office by her parents. They tell you she’s unable to tolerate oral fluids, and vomited twice after being given small amounts of juice and soup earlier in the day. Sarah has decreased urine output, but she is not febrile and has no blood in her stools. On examination, you find mild tachycardia, dry mucous membranes, delayed capillary refill, and normal mental status.
You try giving Sarah an oral electrolyte solution, but she vomits immediately. Her parents are reluctant to take her to the emergency department for intravenous (IV) hydration, and ask if you can provide a safe and effective alternative.
Each year in the United States, pediatric gastroenteritis and dehydration are responsible for approximately 1.5 million outpatient visits2 and 150,000 to 170,000 hospital admissions.3 Oral hydration, recommended by pediatric practice guidelines2,4 and the World Health Organization,5 is safe and generally effective. But, as in Sarah’s case, emesis frequently interferes, leading to hospital admission for IV hydration.
An antiemetic with fewer adverse effects
Older antiemetic medications, such as promethazine, prochlorperazine, and metoclopramide, can cause sedation and extrapyramidal reactions. Ondansetron, a selective 5-hydroxytryptamine (5-HT3) receptor antagonist that has been used to control postoperative and chemotherapy-associated nausea and vomiting in children and adults, does not cause either problem. In recent studies of ondansetron’s effectiveness in treating children with gastroenteritis, increased diarrhea, lasting up to 48 hours after administration, was the only adverse event.1
Two earlier systematic reviews—a meta-analysis by Szajewska et al6 and a Cochrane review7—found clinical benefits of ondansetron for vomiting associated with acute gastroenteritis. But both concluded that the evidence was insufficient to recommend routine use of this drug. The meta-analysis that we review below included additional studies, and the researchers reached a different conclusion.
STUDY SUMMARY: Antiemetic decreases vomiting, hospitalization
DeCamp et al conducted a systematic review and meta-analysis of 11 prospective controlled trials that evaluated antiemetic use in children with vomiting from acute gastroenteritis.1 Six of the 11 trials focused on ondansetron;8-13 these 6 were the most recently published and of the highest quality. (The researchers found the remaining 5 trials to be of low methodological quality, with small sample sizes and inconsistent results, and concluded that the antiemetics they assessed should not be used for outpatients with gastroenteritis.) Their meta-analysis of these 6 trials is the focus of this PURL.
The ondansetron studies included a total of 745 children with vomiting and a clinical diagnosis of gastroenteritis. In 5 of the trials, patients received only 1 dose of ondansetron;8-10,12,13 in the sixth, families received additional doses of ondansetron to use at home.11 In 3 trials, patients were given oral ondansetron—a tablet placed on the tongue that dissolves in minutes. The remaining 3 used an IV formulation.8,10,13 Five trials were conducted in emergency departments (EDs),9-13 and 1 in an inpatient setting.8
Big reductions. Children who received ondansetron had significantly less vomiting (16.9% vs 37.8%) and IV fluid administration (13.9% vs 33.9%), and fewer hospital admissions (7.5% vs 14.6%) compared with patients who were given a placebo (TABLE). Diarrhea, the only adverse event to be systematically evaluated, was assessed in all but 1 of the trials.8-12 In 3 of the 5 that reported on this side effect, patients who received ondansetron had an increase in diarrhea for up to 48 hours.8,11,12
TABLE
Ondansetron reduces vomiting, hospitalization, and IV fluid use
| TOTAL NUMBER OF PATIENTS (N=745) | ONDANSETRON | PLACEBO | RR (95% CI) | NNT (95% CI) |
|---|---|---|---|---|
| Continued vomiting (n=659) | 16.9% | 37.8% | 0.45 (0.33-0.62) | 5 (4-7) |
| IV fluid administration (n=489) | 13.9% | 33.9% | 0.41 (0.28-0.62) | 5 (4-8) |
| Hospital admission (n=662) | 7.5% | 14.6% | 0.52 (0.27-0.95) | 14 (9-44) |
| CI, confidence interval; IV, intravenous; NNT, number needed to treat; RR, relative risk. | ||||
| Source: DeCamp LS, et al. Arch Pediatr Adolesc Med.1 | ||||
WHAT’S NEW: Support for a strategy increasingly used in EDs
Physicians are just beginning to adopt the use of ondansetron as a strategy for avoiding IV hydration and hospitalization for children with vomiting associated with minor gastrointestinal illness. As an adjunct to our report on this meta-analysis, we analyzed the use of the antiemetic in children between the ages of 1 and 10 years in emergency visits reported to the National Ambulatory Medical Care Survey database from 2002 to 2006. Among an estimate of more than 3 million pediatric visits to EDs for acute gastroenteritis in each of these years, in 2002 only 0.53% were treated with ondansetron. By 2006, that percentage had risen to 6.43%.
A similar analysis of both ED and outpatient visits to academic medical centers and teaching hospitals from 2005 through 2008 (estimated using data through October 2008), derived from the University Health System Consortium Clinical Database, showed a similar trend. In 2005, only 0.5% of children presenting to EDs and 0.5% of those seeking outpatient care for acute gastritis received ondansetron. By 2008, the numbers had grown to an estimated 3.43% and 3.60%, respectively.
Given the positive results of the DeCamp study and the fact that oral ondansetron is now available in a generic formulation, we expect the use of this antiemetic to increase in both outpatient and emergency settings. We think quite a few IV lines and hospitalizations could be avoided with the use of this antiemetic, not to mention the symptomatic relief for children.
CAVEATS: Studies didn’t look at milder cases, primary care
None of the studies of oral ondansetron for acute gastroenteritis involved outpatient settings, and all 6 of the trials featured children who were moderately ill. It has not yet been determined whether the benefits seen in the ED will apply to an ambulatory population in which many potential candidates for ondansetron have milder gastroenteritis. Nor is it clear whether oral ondansetron would complement oral rehydration in primary care practices. More detailed evaluation of the reduction of vomiting at home over the course of the illness would help to answer these questions.
Nonetheless, ondansetron appears to be safe. Increased diarrhea, the only documented side effect, resolved after 48 hours, and did not appear to result in higher health care utilization.
Don’t prescribe over the phone. It is important to note that all the ondansetron trials included an evaluation of each patient to consider other etiologies, such as central nervous system disorders or toxic exposures, prior to treatment. Physicians are cautioned not to prescribe antiemetics over the telephone—or without first ruling out more serious illnesses in which vomiting is part of the presentation.
Studies were funded by pharma. The primary studies of ondansetron were funded by GlaxoSmithKline, the pharmaceutical company that manufactures the drug under the trade name Zofran. The authors of the meta-analysis reviewed the Clinical Trials Registry and the reference lists of the articles and contacted other experts to find any unreported trials, but found no evidence of negative publication bias. Therefore, we have confidence in these findings. Ideally, additional studies will be conducted without drug company support, in an outpatient setting, to clarify the use of ondansetron as an adjunct to oral rehydration.
CHALLENGES TO IMPLEMENTATION: No major barriers
Cost should not be a barrier to the use of oral ondansetron. The generic formulation sells for $10 to $20 per tablet, and is covered by most health insurers. However, treatment of children with acute gastroenteritis and moderate dehydration in the office setting would likely require a period of observation for tolerance of oral rehydration before and after administration of ondansetron. This may be impractical in some busy clinics.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Give oral ondansetron to children with acute gastroenteritis and moderate dehydration who are unable to tolerate oral rehydration to reduce the vomiting and avoid the need for intravenous (IV) hydration or hospitalization.1
Strength of recommendation
A: Meta-analysis of 6 high-quality studies
DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
ILLUSTRATIVE CASE
Sarah, a 2-year-old who has been vomiting and had diarrhea for the past 2 days, is brought to your office by her parents. They tell you she’s unable to tolerate oral fluids, and vomited twice after being given small amounts of juice and soup earlier in the day. Sarah has decreased urine output, but she is not febrile and has no blood in her stools. On examination, you find mild tachycardia, dry mucous membranes, delayed capillary refill, and normal mental status.
You try giving Sarah an oral electrolyte solution, but she vomits immediately. Her parents are reluctant to take her to the emergency department for intravenous (IV) hydration, and ask if you can provide a safe and effective alternative.
Each year in the United States, pediatric gastroenteritis and dehydration are responsible for approximately 1.5 million outpatient visits2 and 150,000 to 170,000 hospital admissions.3 Oral hydration, recommended by pediatric practice guidelines2,4 and the World Health Organization,5 is safe and generally effective. But, as in Sarah’s case, emesis frequently interferes, leading to hospital admission for IV hydration.
An antiemetic with fewer adverse effects
Older antiemetic medications, such as promethazine, prochlorperazine, and metoclopramide, can cause sedation and extrapyramidal reactions. Ondansetron, a selective 5-hydroxytryptamine (5-HT3) receptor antagonist that has been used to control postoperative and chemotherapy-associated nausea and vomiting in children and adults, does not cause either problem. In recent studies of ondansetron’s effectiveness in treating children with gastroenteritis, increased diarrhea, lasting up to 48 hours after administration, was the only adverse event.1
Two earlier systematic reviews—a meta-analysis by Szajewska et al6 and a Cochrane review7—found clinical benefits of ondansetron for vomiting associated with acute gastroenteritis. But both concluded that the evidence was insufficient to recommend routine use of this drug. The meta-analysis that we review below included additional studies, and the researchers reached a different conclusion.
STUDY SUMMARY: Antiemetic decreases vomiting, hospitalization
DeCamp et al conducted a systematic review and meta-analysis of 11 prospective controlled trials that evaluated antiemetic use in children with vomiting from acute gastroenteritis.1 Six of the 11 trials focused on ondansetron;8-13 these 6 were the most recently published and of the highest quality. (The researchers found the remaining 5 trials to be of low methodological quality, with small sample sizes and inconsistent results, and concluded that the antiemetics they assessed should not be used for outpatients with gastroenteritis.) Their meta-analysis of these 6 trials is the focus of this PURL.
The ondansetron studies included a total of 745 children with vomiting and a clinical diagnosis of gastroenteritis. In 5 of the trials, patients received only 1 dose of ondansetron;8-10,12,13 in the sixth, families received additional doses of ondansetron to use at home.11 In 3 trials, patients were given oral ondansetron—a tablet placed on the tongue that dissolves in minutes. The remaining 3 used an IV formulation.8,10,13 Five trials were conducted in emergency departments (EDs),9-13 and 1 in an inpatient setting.8
Big reductions. Children who received ondansetron had significantly less vomiting (16.9% vs 37.8%) and IV fluid administration (13.9% vs 33.9%), and fewer hospital admissions (7.5% vs 14.6%) compared with patients who were given a placebo (TABLE). Diarrhea, the only adverse event to be systematically evaluated, was assessed in all but 1 of the trials.8-12 In 3 of the 5 that reported on this side effect, patients who received ondansetron had an increase in diarrhea for up to 48 hours.8,11,12
TABLE
Ondansetron reduces vomiting, hospitalization, and IV fluid use
| TOTAL NUMBER OF PATIENTS (N=745) | ONDANSETRON | PLACEBO | RR (95% CI) | NNT (95% CI) |
|---|---|---|---|---|
| Continued vomiting (n=659) | 16.9% | 37.8% | 0.45 (0.33-0.62) | 5 (4-7) |
| IV fluid administration (n=489) | 13.9% | 33.9% | 0.41 (0.28-0.62) | 5 (4-8) |
| Hospital admission (n=662) | 7.5% | 14.6% | 0.52 (0.27-0.95) | 14 (9-44) |
| CI, confidence interval; IV, intravenous; NNT, number needed to treat; RR, relative risk. | ||||
| Source: DeCamp LS, et al. Arch Pediatr Adolesc Med.1 | ||||
WHAT’S NEW: Support for a strategy increasingly used in EDs
Physicians are just beginning to adopt the use of ondansetron as a strategy for avoiding IV hydration and hospitalization for children with vomiting associated with minor gastrointestinal illness. As an adjunct to our report on this meta-analysis, we analyzed the use of the antiemetic in children between the ages of 1 and 10 years in emergency visits reported to the National Ambulatory Medical Care Survey database from 2002 to 2006. Among an estimate of more than 3 million pediatric visits to EDs for acute gastroenteritis in each of these years, in 2002 only 0.53% were treated with ondansetron. By 2006, that percentage had risen to 6.43%.
A similar analysis of both ED and outpatient visits to academic medical centers and teaching hospitals from 2005 through 2008 (estimated using data through October 2008), derived from the University Health System Consortium Clinical Database, showed a similar trend. In 2005, only 0.5% of children presenting to EDs and 0.5% of those seeking outpatient care for acute gastritis received ondansetron. By 2008, the numbers had grown to an estimated 3.43% and 3.60%, respectively.
Given the positive results of the DeCamp study and the fact that oral ondansetron is now available in a generic formulation, we expect the use of this antiemetic to increase in both outpatient and emergency settings. We think quite a few IV lines and hospitalizations could be avoided with the use of this antiemetic, not to mention the symptomatic relief for children.
CAVEATS: Studies didn’t look at milder cases, primary care
None of the studies of oral ondansetron for acute gastroenteritis involved outpatient settings, and all 6 of the trials featured children who were moderately ill. It has not yet been determined whether the benefits seen in the ED will apply to an ambulatory population in which many potential candidates for ondansetron have milder gastroenteritis. Nor is it clear whether oral ondansetron would complement oral rehydration in primary care practices. More detailed evaluation of the reduction of vomiting at home over the course of the illness would help to answer these questions.
Nonetheless, ondansetron appears to be safe. Increased diarrhea, the only documented side effect, resolved after 48 hours, and did not appear to result in higher health care utilization.
Don’t prescribe over the phone. It is important to note that all the ondansetron trials included an evaluation of each patient to consider other etiologies, such as central nervous system disorders or toxic exposures, prior to treatment. Physicians are cautioned not to prescribe antiemetics over the telephone—or without first ruling out more serious illnesses in which vomiting is part of the presentation.
Studies were funded by pharma. The primary studies of ondansetron were funded by GlaxoSmithKline, the pharmaceutical company that manufactures the drug under the trade name Zofran. The authors of the meta-analysis reviewed the Clinical Trials Registry and the reference lists of the articles and contacted other experts to find any unreported trials, but found no evidence of negative publication bias. Therefore, we have confidence in these findings. Ideally, additional studies will be conducted without drug company support, in an outpatient setting, to clarify the use of ondansetron as an adjunct to oral rehydration.
CHALLENGES TO IMPLEMENTATION: No major barriers
Cost should not be a barrier to the use of oral ondansetron. The generic formulation sells for $10 to $20 per tablet, and is covered by most health insurers. However, treatment of children with acute gastroenteritis and moderate dehydration in the office setting would likely require a period of observation for tolerance of oral rehydration before and after administration of ondansetron. This may be impractical in some busy clinics.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors wish to acknowledge Sofia Medvedev, PhD, of the University HealthSystem Consortium in Oak Brook, Ill, for analysis of the National Ambulatory Medical Care Survey data and the UHC Clinical Database.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
3. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97:424-435.
5. World Health Organization. Clinical management of acute diarrhoea. WHO/Unicef joint statement. Available at http://www.who.int/child_adolescent_health/documents/who_fch_cah_04_7/en/index.html. Accessed January 15, 2009.
6. Szajewska H, Gieruszczak-Bialek D, Dylag M. Meta-analysis: ondansetron for vomiting in acute gastroenteritis in children. Aliment Pharmacol Ther. 2007;25:393-400.
7. Alhashimi D, Alhashimi H, Fedorowicz Z. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2006;(4):CD005506.-
8. Cubeddu LX, Trujillo LM, Talmaciu I, et al. Antiemetic activity of ondansetron in acute gastroenteritis. Aliment Pharmacol Ther. 1997;11:185-191.
9. Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52:22-29.
10. Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis; a randomized, controlled trial. Pediatrics. 2002;109:e62.-Available at: http://pediatrics.aappublications.org/cgi/reprint/109/4/e62. Accessed January 12, 2009.
11. Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med. 2002;39:397-403.
12. Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354:1698-1705.
13. Stork CM, Brown KM, Reilly TH, et al. Emergency department treatment of viral gastritis using intravenous ondansetron or dexamethasone in children. Acad Emerg Med. 2006;13:1027-1033.
1. DeCamp LS, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis, a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858-865.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
3. Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887-1892.
4. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97:424-435.
5. World Health Organization. Clinical management of acute diarrhoea. WHO/Unicef joint statement. Available at http://www.who.int/child_adolescent_health/documents/who_fch_cah_04_7/en/index.html. Accessed January 15, 2009.
6. Szajewska H, Gieruszczak-Bialek D, Dylag M. Meta-analysis: ondansetron for vomiting in acute gastroenteritis in children. Aliment Pharmacol Ther. 2007;25:393-400.
7. Alhashimi D, Alhashimi H, Fedorowicz Z. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2006;(4):CD005506.-
8. Cubeddu LX, Trujillo LM, Talmaciu I, et al. Antiemetic activity of ondansetron in acute gastroenteritis. Aliment Pharmacol Ther. 1997;11:185-191.
9. Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52:22-29.
10. Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis; a randomized, controlled trial. Pediatrics. 2002;109:e62.-Available at: http://pediatrics.aappublications.org/cgi/reprint/109/4/e62. Accessed January 12, 2009.
11. Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med. 2002;39:397-403.
12. Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354:1698-1705.
13. Stork CM, Brown KM, Reilly TH, et al. Emergency department treatment of viral gastritis using intravenous ondansetron or dexamethasone in children. Acad Emerg Med. 2006;13:1027-1033.
Copyright © 2009 The Family Physicians Inquiries Network.
All rights reserved.