Article
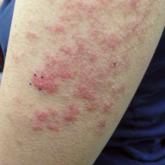
Bullous Systemic Lupus Erythematosus Successfully Treated With Rituximab
- Author:
- Christopher D. Lowe, MD
- Catherine A. Brahe, MD
- Brian Green, DO
- Thomas K. Lam, MD
- Jon H. Meyerle, MD
Bullous systemic lupus erythematosus can present with a waxing and waning course punctuated by flares. This case illustrates the role of rituximab...
Article
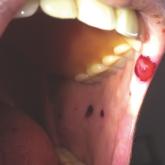
Heparin-Induced Bullous Hemorrhagic Dermatosis Confined to the Oral Mucosa
- Author:
- Hannah B. Harris, MD
- Benjamin J. Kurth, MD
- Thomas K. Lam, MD
- Jon H. Meyerle, MD
It is important for physicians to recognize the clinical appearance of cutaneous adverse reactions to heparin, including bullous hemorrhagic...
