User login
Large Healthcare System VTE Prevention
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
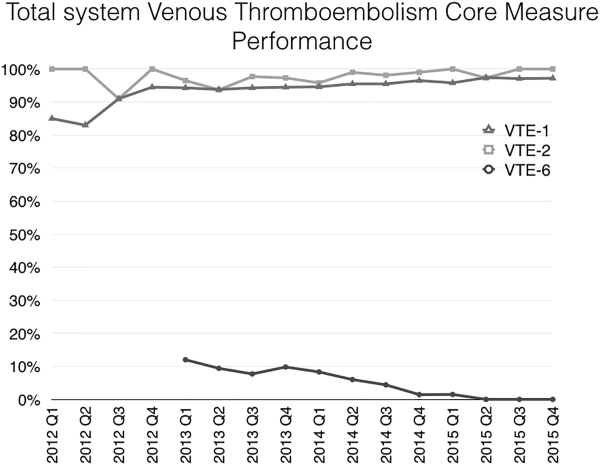
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.
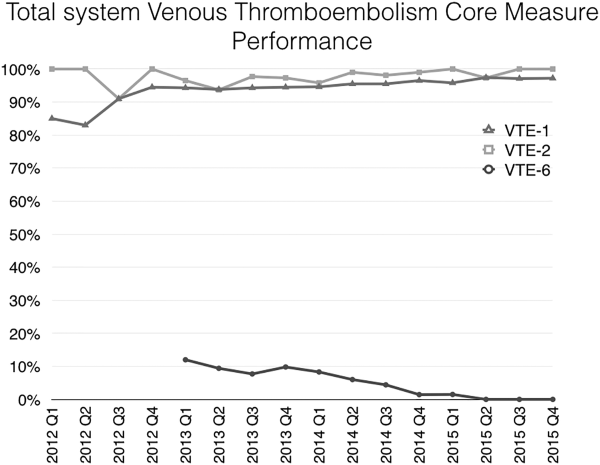
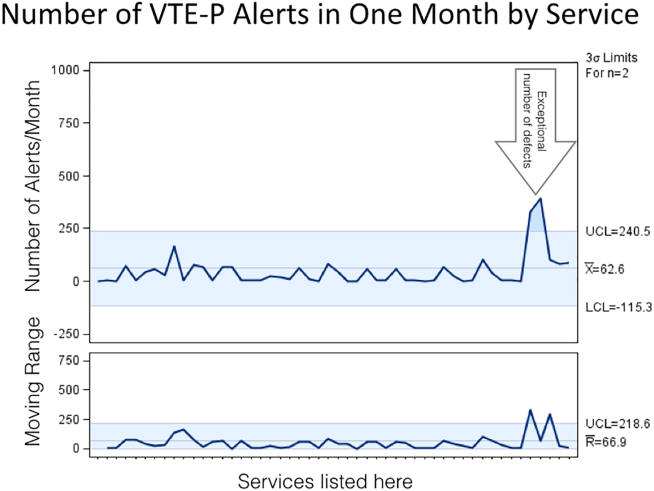
Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.
Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.

Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.

Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
Venous thromboembolism (VTE), including both deep vein thrombosis (DVT) and pulmonary embolism, is a major cause of preventable hospital death and long‐term morbidity. VTE accounts for approximately 100,000 to 200,000 hospital deaths annually,[1] and preventable DVT costs an estimated $2.5 billion annually, with each case resulting in direct hospital costs of an estimated $25,977.[2] Although VTE is less common in children, its incidence is increasing in the medically ill hospitalized pediatric patient. The most recent analysis of a large national children's hospital database showed VTE rates increasing from 34 to 58 per 10,000 admissions from 2001 to 2007.[3] Rates in pediatric trauma patients are higher, at 60 to 100 per 10,000 admissions.[4, 5, 6]
The Joint Commission, the Surgeon General, and the Centers for Disease Control and Prevention have supported initiatives to increase awareness and promote strategies designed to prevent hospital acquired VTE.[7, 8] There are several high‐quality, evidence‐based VTE prophylaxis (VTE‐P) guidelines for adult hospitalized populations.[9, 10, 11] Pediatric VTE‐P guidelines are not well established, but the literature regarding VTE risk stratification and prophylaxis guidelines for medically complex children is growing.[12, 13, 14, 15, 16, 17]
A significant challenge has been developing systems that ensure that evidence and consensus‐based care recommendations are reliably implemented. This summary will describe the methods applied across an integrated health system that includes 22 acute care facilities and 1 pediatric hospital across 5 states that have resulted in a significant reduction in preventable VTE.
SETTING
Mayo Clinic is an integrated health system that owns 22 acute care facilities across 5 states, housing 3971 beds with approximately 122,000 admissions per year. Mayo Clinic Rochester, Arizona, and Florida are all tertiary academic medical centers with trauma and transplant programs. During this project, Arizona and Florida utilized a common build of the Cerner (Kansas City, MO) electronic health record (EHR). The other facilities, collectively referred to as the Mayo Clinic Health System (MCHS) hospitals, include 1 level II trauma center and 11 critical access hospitals and serve communities of varying sizes in Minnesota, Wisconsin, and Iowa. A different build of the Cerner EHR served the MCHS during this project.
Mayo Clinic Rochester is responsible for nearly 50% of all admissions and procedures. Mayo Eugenio Litta Children's Hospital, Rochester, Minnesota is a tertiary children's hospital facility housing 44 general pediatric beds, 26 neonatal intensive care unit beds, 24 intermediate nursery special care beds, and 16 pediatric intensive care unit beds. Mayo Clinic Rochester used the GE Centricity (GE Healthcare, Wauwatosa, WI) EHR and custom‐designed computerized decision support.
METHODS
Mayo Clinic has developed a system to deliberately speed the diffusion of best practices across our system to drive reliable, evidence‐based care, reduce unwanted variation in processes and outcomes, and improve value.[18] The 3 main components of this system are (1) discovery: wherein we learn a practice that demonstrably solves the clinical problem well in at least 1 of our facilities, (2) assessment of readiness for diffusion, and (3) diffusion of the best practice across all of Mayo Clinic. The diffusion process is active and equipped with a project team and execution timeline. Both adult and pediatric projects began with discovery phases. The adult program has fully diffused; the pediatric program is diffusing at this writing.
ADULT ACUTE CARE PATIENTS
Discovery Through Pilot Projects
Beginning in 2006, 2 spontaneously convened interdisciplinary teams worked independently in selected medical and surgical practices in our Rochester hospital to improve VTE‐P. Each team's work resulted in the reduction of defect rates on pilot hospital services to <10%. Key findings were: (1) the vast majority of patients in the pilot had at least 1 risk factor for VTE and (2) when physicians explicitly determined a VTE‐P plan, they made the correct decision 98% of the time without any specific risk rule or point system.[18] Both teams found that efforts to ensure declaration of VTE‐P plans in the workflow of admission resulted in the most improvement in appropriate VTE‐P rates.
Creation of VTE‐Prevention Plans and the VTE Prophylaxis Tollgate
Based on lessons learned from the pilot projects, multidisciplinary improvement teams focused on adaptation of optimal VTE‐P plans for individual practices (eg, preferred VTE‐P for a neurosurgery patient is not the same as for a medical patient), and a VTE‐P tollgatea requirement for providers to complete a VTE‐P plan for each patientwas integrated into the clinical workflow of all order sets used for admissions, transfers, and for selected postoperative order sets. As we moved from the paper systems to computerized order entry, tollgates were subsequently converted to the GE Centricity electronic environment. To minimize burden on clinicians, designs were tested in a usability laboratory prior to operational deployment to ensure that they were as clear and easy as our software would allow.
Alerts
Based on initial reports and feedback, our clinical decision support (CDS) team designed alerts that notify the clinician when (1) any patient previously declared as at least moderate risk for VTE did not have a valid VTE‐P plan in place for any 24‐hour period, or (2) when any patient carried a low‐risk categorization for >3 days (because this should prompt reconsideration of risk status). Alerts were designed to be clear and to facilitate steps to correct the situation.
VTE‐P alerts would present to any member of the patient's provider team who accessed the patient's EHR, and would continue to alert with each access until conditions were rendered to satisfy the requirements of the alert. Each alert provided easy access to an abbreviated VTE‐P tollgate order that would allow the provider to select a clinically appropriate response: either restate the low‐risk status, change the low‐risk status and add an active VTE‐P order, specify why neither mechanical nor pharmacologic VTE‐P may be given, or restart a VTE‐P order.
Monitoring
Both process and outcome measures were used to monitor the effectiveness of VTE‐P activities. During initial roll‐out, the teams measured and reported the proportion of patients where either (1) VTE risk factors were present (patient is determined to be at least at moderate risk for VTE) and either pharmacologic or mechanical VTE‐P was ordered within 24 hours of admission, or (2) VTE risk factors were not present, and VTE‐P not indicated was documented within 24 hours of admission. The CDS system also provided ongoing monitoring of CDS‐alert firing frequency, which closely correlated with the prevalence of patients without a valid VTE‐P plan.
Diffusion Across All Units of Mayo Rochester
Diffusion teams included physician champions, project managers, a pharmacist, and a nurse. To emphasize the engagement of institutional leadership, the project was commissioned by the institution's Clinical Practice Quality Oversight Committee and co‐chaired by the Department of Medicine Associate Chair for Quality and the Chair of the Surgical Quality and Safety Subcommittee.
Implementation of this integrated system resulted in substantial improvement to 97% hospital‐wide VTE‐P rates that were sustained over 3 quarters. At that time, a decision was made to diffuse this new best practice across all Mayo Clinic acute care facilities.
Diffusion to All 22 Mayo Clinic Acute Care Facilities
After readiness for diffusion assessment,[18] an enterprise diffusion team, this time led by the Mayo Clinic Patient Safety Officer (an MD), 3 other physician champions (1 from each region of the Mayo Clinic), a project manager, a pharmacist, a computerized physician order entry system content specialist, and the institutional quality office personnel who assisted with the measurement, analysis, and display of data at the work sites. The best practices diffused were: (1) All admission or transfer order sets will have a VTE‐P tollgate. (2) All VTE‐P tollgates will be a force function (ie, they cannot be bypassed). (3) Over 95% of all eligible patients in the facility at any given moment will have a valid VTE‐P plan in place. (4) Ongoing compliance monitoring must be available as an automatic feed, not by chart review. The end goal of our diffusion process was to ensure that all best practices were ensured at all of our facilities.
Key issues in the diffusion process included implementation of the VTE‐P tollgates into all admission or transfer order sets and the computer decision support logic that had been developed in the GE Centricity system into the Cerner EHR. Each system had slightly different constraints to 4 best practices to be diffused. We had difficulty designing the GE Centricity order sets or flags in such a way that absolutely forced an action (best practice items 1 and 2). Instead, our design had to alert the ordering provider until the appropriate conditions were met. This is suboptimal in that it creates the potential for alarm fatigue and subsequent error. It is for that reason that a tight monitoring system was necessary to provide feedback on a per‐provider level if the alerts were too numerous (suggesting that alarm fatigue or misunderstanding might be leading to failure to correct the unsafe situation producing the alarm).
In contrast, the Cerner system did not have as much capability for our IT support to provide as much customization of decision support but was fully capable of forcing functions. Therefore, we needed to provide a more rigid logic into the order sets. This led to a less than optimal user interface each time a patient was admitted or transferred, but fulfilled mission goals.
Pediatric Patients
Pediatric Discovery Project
Development of Pediatric VTE Risk‐Assessment Tool
To develop a VTE‐P system for our pediatric hospital, our first task was to design a VTE risk‐stratification tool. The improvement team included a physician, pharmacist, and clinical nurse specialists from pediatric intensive care unit (PICU), cardiac intensive care units, and general pediatric services. A literature review identified the most common published risk factors for VTE in children. We next performed a retrospective review of pediatric hospital‐acquired VTE in 2011 to 2012. Eight VTEs were identified (infants to age 18 years). All were related to central venous catheters, sepsis, congenital heart disease, leukemia, myocarditis, and extreme prematurity (Table 1). In contrast to other series, our patients were younger (80% less than 14 years of age). Based on these reviews and iterative consensus with our pediatric staff, an initial pediatric VTE risk‐screening tool was designed and piloted first in the PICU for usability and to assess face validity.
| Age/Gender | Main Diagnosis | Comorbidities | Central Lines Prior to VTE Event | VTE Event |
|---|---|---|---|---|
| ||||
| 18 y/M | Congenital heart disease | Heart transplant | Right and left IJV, right arterial, left femoral vein and artery | DVT left IJV, innominate, subclavian, axillary veins |
| 3 y/M | Idiopathic myocarditis | ECMO | Left radial arterial, right brachial PICC, RIJ venous, left arterial femoral, right venous femoral, | Cerebral embolism with multiple infarcts |
| 0.2 y/F | Premature | NEC | Right IJ PICC | Right axillary, subclavian DVT, left greater saphenous vein |
| 0.5 y/M | Premature | Hypoxic‐ischemic encephalopathy | Umbilical artery and vein catheters, right IJ PICC, right femoral venous | IVC thrombosis and bilateral renal veins |
| 0.1 y/F | Sepsis | RSV pneumonia | Right femoral venous | Right common femoral and right external iliac DVT |
| 12 y/M | Acute lymphoblastic leukemia | Renal dysfunction | Left femoral arterial, right femoral venous | Right common femoral DVT |
| 0.1 y/M | Congenital heart disease | Umbilical artery and vein catheters, left femoral artery, right IJV | Left femoral artery thrombosis | |
| 1 y/M | Seizures | Partially treated meningitis/hyponatremia | Left femoral vein | Left common femoral and external iliac DVT |
Developing Consensus About Appropriate VTE‐P
The risk of even low‐dose anticoagulation may be higher in children than in adults. Therefore, in addition to first estimating the risk for VTE, we also incorporated into the risk‐assessment tool an estimate of risk for bleeding (Table 2). Physicians were responsible for using the VTE‐P screening. Bleeding risk‐assessment categories included: intracranial bleed, premature infant, internal injury (eg, organ injury, splenic laceration), planned surgery within 24 hours, renal failure, liver dysfunction, coagulopathy, thrombocytopenia (eg, platelets <50,000), disseminated intravascular coagulation, congenital bleeding disorder, and neurosurgical and spine fusion patients. If any of these were present, pharmacologic prophylaxis was contraindicated. If a patient was considered at risk for VTE, a pediatric hematology consult was recommended or advised. If there was no increased bleeding risk and the child had 2 or more risk factors or a central venous catheter with additional thrombosis risk factors, the consensus was to use appropriately dosed low‐molecular‐weight heparin or unfractionated heparin in addition to mechanical prophylaxis. A patient considered at increased risk for bleeding but with risk factors for thrombosis would receive early ambulation and/or mechanical prophylaxis. In all cases, removal of central catheters was recommended within 72 hours if possible.
|
| Risk factors |
| Central venous catheter 7 days |
| High‐risk orthopedic surgery |
| Complex fracture of pelvis or lower extremity |
| Projected immobility for 7 days |
| History of prior VTE |
| History of prior thrombophilia |
| ECMO |
| Malignancy |
| Multiple body trauma |
| Use of hormonal therapy |
| BMI > 95th percentile |
| Continuous BPAP/CPAP or mechanical ventilation |
| Inflammatory bowel disease |
| Guidance if no increased bleeding risk |
| 2 risk factorsmechanical combined with pharmacologic prophylaxis |
| Central venous catheter 7 days and additional thrombosis risk factorsmechanical combined with pharmacologic prophylaxis |
| Pharmacologic prophylaxis generally not utilized in spine or neurosurgery patients |
| Guidance if increased bleeding risk |
| 2 risk factors or central venous catheter and additional thrombosis risk factors 7 days hematology consult |
| 2 risk factors or central venous catheter 7 days and additional thrombosis risk factorsearly ambulation + mechanical VTE‐P |
Pilot Implementation
We initiated use of the risk‐assessment tool and VTE‐P algorithm in the PICU using a paper system at first, and measured via chart review (1) the proportion of patients for whom a VTE‐P risk assessment was completed according to the recommended plan and (2) the proportion with the appropriate VTE‐P plan selected based upon risk factors present. The risk‐assessment tool was iteratively improved and built into the electronic order system (Table 2). This would ensure diffusion across the children's hospital, and would be subsequently diffused across the rest of Mayo Clinic.
Metrics
During the system diffusion for the adult system, we relied on 2 metrics to measure improvement: the CDS alert frequency and Centers for Medicare and Medicaid Services (CMS) VTE Core Measures. The CDS alert frequency is cross‐sectional and can be used to estimate what percentage of patients at any given moment in time in our hospital have a valid VTE‐P. From chart audits, we anticipate that at target, approximately 4% of patients would generate CDS alerts because needs and plans change in the dynamic care environment. For example, VTE‐P may be held for a procedure, or during transition from 1 to another unit. Or, observation patients may have been classified as low risk, but when converted to admission status there may be a lag while the VTE risk status is changed. These data can be provided by service and provider, and are reported back to the providers to help reduce practice variation.
In addition, the CMS Core Measures provided a manual chart review metric to supplement the automated data. VTE‐1 and VTE‐2 measures the proportion of sampled charts demonstrating either delivery of VTE‐P or declaration of low risk in non‐ICU and ICU patients, respectively. VTE‐6, the proportion of patients acquiring a VTE who did not receive prophylaxis, served as our outcome measure. For the pediatric efforts, manual chart review served during the improvement pilots, but will be supplanted by a similar automated system.
RESULTS
Adult Acute Care Patients
Mayo Clinic used CMS Core Measures in all 22 hospitals in the system from 2013 onward. The results are shown in Figure 1. Of note, VTE‐1 has improved from its project start values in the mid‐80% range to consistently above 95% for the last 6 quarters (most recently above 97%), VTE‐2 has averaged 97.3%, and most recently is at 100%, and VTE‐6 has declined from about 12% to 0% in the recent quarters.

Figure 2 shows the number of VTE‐P alerts generated during 1 month by service in Mayo Clinic Rochester. We display these data as control charts so that practices on services with a statistically excessive number of alerts can be targeted for improvement. Similar data are available at all institutions.

Pediatric Patients
The PICU had an average of 101 admissions per month during study period (range, 72120) with a mean of 11 patients per day (range, 912 patients). Prior to the VTE‐P pilot, none had VTE risks documented. A total of 773 patients were screened for VTE in the intensive care unit during the study period, of which 194 were identified with 2 or greater VTE risk factors (25%). Sixty‐six of 194 patients (34%) had pharmacologic and/or mechanical prophylaxis (n = 83, 44%) selected for VTE‐P. No bleeding events were reported among these patients. During the discovery pilot, the VTE screening tool resulted in >92% compliance with risk documentation, >64% appropriate VTE‐P use, and 0 VTE events. The subsequently improved screening tool resulted in approximately 88% compliance over the subsequent 6 months of use, and in 9 months 2 VTE were diagnosed (both occurring in hospital units not using the screening tool).
An electronic VTE‐P tollgate for pediatric patients went live on March 17, 2016 (Figure 3). We have also developed a CDS alert for pediatric patients not having an appropriate VTE‐P plan documented, and alert frequency reports will allow focused improvement efforts if needed.

DISCUSSION
Our VTE‐P system has resulted in significant reductions in preventable VTE. The key components of our system are: (1) Ensure that a VTE‐P is declared at admission by providing a mandatory VTE‐P tollgate that requires the provider to assess the risk for VTE and provide an appropriate order for VTE‐P. (2) Use clinical decision support to provide ongoing surveillance and alerting providers when there is a lapse in the VTE‐P plan. With these, we have driven CMS Core Measures VTE‐6 to 0 over 3 quarters.
Different VTE‐P strategies have been implemented among hospitalized medically ill patients. Despite the morbidity and mortality risks inherent to VTE, some studies have shown that more than half and nearly 79% of high‐risk hospitalized medical patients received no VTE prevention.[19] Among those who received prophylactic therapy, inadequate duration or type was prescribed in nearly 44%.[20] Electronic orders have resulted in improved prophylaxis in some literature reports.[21, 22] One study showed that a physician alert reduced VTE incidence from 4.13 to 2.23 events per 10,000 patients.[21] Our system, combining prompted electronic orders with clinical decision support for ongoing real‐time monitoring for VTE‐P plans appears to have been effective in producing reliable ordering of VTE‐P in both adults and children.
However, our system has limitations, some inherent in its design and others not addressed yet. Intrinsically, we depend upon clinicians to rightly gauge the patient risk for VTE‐P. Because a significant majority of our patients have at least moderate risk for VTE, the construction of the order sets tend to guide the clinician to select some form of prophylaxis. However, our system does not specifically provide guidance as to what VTE‐P to choose. If the clinician deems the patient at low risk, the CDS criteria will accept this judgment for up to 3 days without questioning the provider. Similarly, by national criteria, some patients at very high risk would ideally receive both mechanical and pharmacologic VTE‐P.[23] Our monitoring system does not distinguish between very high risk and moderately high risk when determining if a valid VTE‐P is in place. Audits of clinician decision making have shown that at present the appropriate decisions are being made 98% of the time, but this could change over time and with new guideline recommendations. Another challenge concerns the difference between ordering and delivering prophylaxis. When ordered, pharmacologic VTE‐P is reliably delivered. In contrast, providing ongoing delivery of ordered mechanical VTE‐P is more challenging. In addition, our current system does not extend to VTE‐P plans for discharge. Future clinical decision support might suggest which patients should receive combined prophylaxis while in the hospital or which home‐going prophylaxis plans should be considered.
We acknowledged the limitations of diffusing a VTE and bleeding risk‐assessment tool that has not been validated in our hospitalized pediatric population. Validation of pediatric VTE risk assessment tools have been recently developed but not widely validated in large prospective studies to be considered the standard of care.[6, 12] Based on our own institutional experience, the vast majority of VTE events occurred in pediatric patients with a central venous catheter (CVC) and other risk factors for thrombosis, and this category was arbitrarily chosen as one to consider pharmacologic prophylaxis if no bleeding risk factors and a central line to be in placed greater than 7 days duration. Although, pediatric evidence guidelines do not support the use of pharmacologic prophylaxis in patients with CVC,[15] risk factors for thrombosis in children, although less frequent than adults, are still present, and VTE‐P should be assessed and individualized in each patient considered at risk for thrombosis. Other groups have attempted a similar approach as the one taken by our group, with variations in the criteria used for thromboprophylaxis in the pediatric population.[5, 14] Our data illustrate that not all pediatric patients require pharmacologic prophylaxis (34%), and VTE‐P should be individualized based on patient risk factors for thrombosis and bleeding risk.
A strength of our system is derived from the substantial clinician and expert input, the codification of consensus, and the hard wiring of that consensus into the electronic ordering, clinical decision rules, and reporting environment. As new advances to VTE‐P are developed, we will strive to codify those new processes into our workflow, building on our past success.
Acknowledgements
The authors acknowledge all of the members involved in the VTE prevention effort at Mayo Clinic including nurses, pharmacists, and information technology support staff.
Disclosure: Nothing to report.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
- U.S. Department of Health and Human Services. Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178. Published 2008. Accessed May 23, 2016.
- , , , , . Deep‐vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415.
- , , , . Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008.
- , , , , . Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52:922–927.
- , , , , , . Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68:52–56.
- , , , , , . Risk factors for in‐hospital venous thromboembolism in children: a case‐control study employing diagnostic validation. Haematologica. 2012;97:509–515.
- . Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123(4):420–421.
- . Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;14(10):901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88:545–549.
- , , , et al. Risk‐prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds‐Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–1334.
- , , , et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297.
- , , , . Thromboprophylaxis in a pediatric hospital: a patient‐safety and quality‐improvement initiative. Pediatrics. 2011;127:e1326–e1332.
- , , , et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S.
- , , , et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta‐analysis of single‐arm studies. Semin Thromb Hemost. 2011;37:814–825.
- , , , . Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35:e287–e291.
- , , , et al. Accelerating the use of best practices: the Mayo Clinic Model of Diffusion. Jt Comm J Qual Patient Saf. 2013;39:167–176.
- , , , , . Disease burden and unmet needs for prevention of venous thromboembolism in medically ill patients in Europe show underutilisation of preventive therapies. Thromb Haemost. 2011;106:600–608.
- , , , . Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement. J Gen Intern Med. 2002;17:788–791.
- , , , et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost. 2008;100:699–704.
- , , , et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. J Intern Med. 2004;256:338–348.
- , , , et al. Low‐molecular‐weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:26: 2463–2472.
© 2016 Society of Hospital Medicine
Increased Falls Associated with Zolpidem
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).
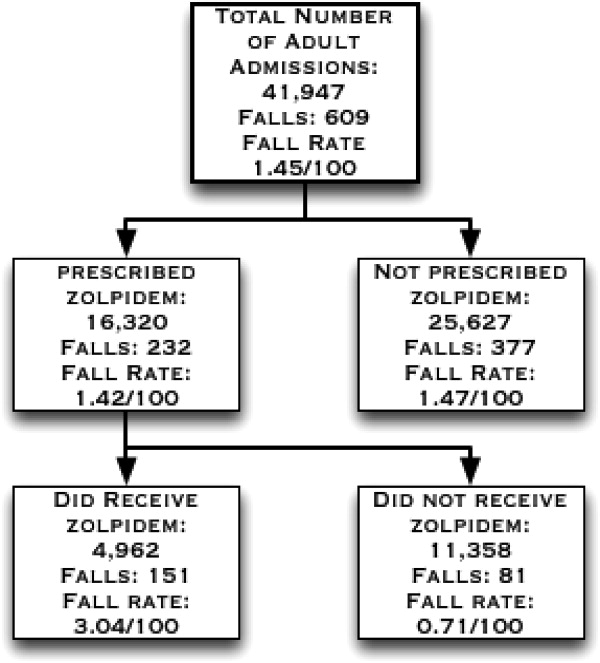
Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).

Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).

Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
Copyright © 2012 Society of Hospital Medicine