User login
Apps and fitness trackers that measure sleep: Are they useful?
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
KEY POINTS
- Wearable fitness trackers tend to perform better than smartphone applications, which are more prone to interference from bed partners and pets.
- Sleep data from tracking devices are less reliable in patients with fragmented sleep and insomnia.
- In normal sleepers, devices tend to measure sleep duration with reasonable accuracy, so that one can tell if a patient is getting too little sleep or reassure someone who is getting enough sleep.
- Devices may help identify patients with poor sleep hygiene or atypical circadian rhythms.
Increased Falls Associated with Zolpidem
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
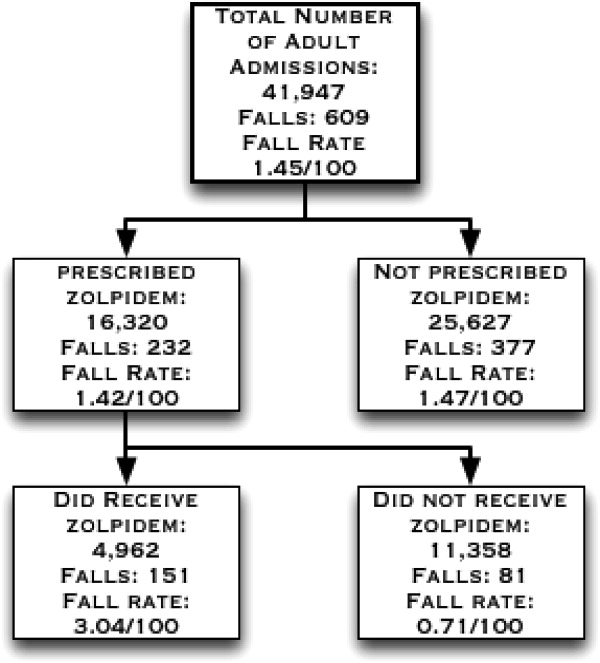
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).
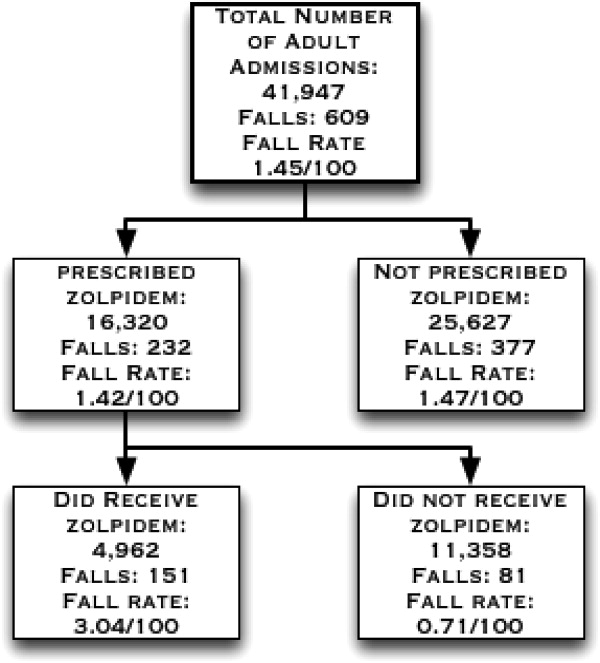
Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).

Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
Hospitalized patients have increased rates of sleep disturbances.1, 2 Sleep disturbances are perceived to be disruptive to both patients and staff, a putative reason for the high rates of hypnotic use in hospitalized patients.3, 4 Zolpidem, a short‐acting, non‐benzodiazepine, benzodiazepine receptor agonist that acts at the ‐aminobutyric acid (GABA)‐A receptor complex, is the most commonly prescribed hypnotic agent in the United States.5, 6 It is also extremely commonly used in inpatient settings. Although zolpidem is thought to have a relatively benign side‐effect profile, it has been found to impair balance in healthy volunteers, even after a single dose.7 Zolpidem use has been found to be higher in community‐dwelling adults who sustained a hip fracture.8, 9
Falls in the inpatient setting are associated with significantly increased morbidity, serious injury, and can result in a prolonged hospital stay and increased healthcare expenditure.10, 11 It is for these reasons that fall reduction is one of the target aims of the Department of Health and Human Services Partnership for Patients.10 While many fall prevention programs have been shown to be effective, they are resource intensive.11 If zolpidem use were associated with increased rates of falls in hospitalized patients, decreasing zolpidem prescription could be an easy and effective intervention in order to reduce fall risk.
A previous case‐control study showed increased zolpidem use in geriatric inpatients who sustained a fall.8 However, the literature linking zolpidem use with an increased fall risk in hospitalized patients is based upon a small sample and does not correct for potential confounders, such as other medication use, delirium, or insomnia.8
We aimed to conduct a cohort study in a large inpatient teaching hospital to ascertain whether zolpidem is associated with increased rates of falls after accounting for age, sex, insomnia, delirium, and use of other medications previously shown to be associated with increased fall risk.
METHODS
All inpatients 18 years or older, admitted in 2010 to hospitals at Mayo Clinic, Rochester, MN, who were prescribed zolpidem were eligible for inclusion in the study. We excluded all patients who were pregnant and those in the intensive care unit (ICU) setting. We compared the group that was prescribed zolpidem and received it, to the group that was prescribed zolpidem but did not receive the medication. We restricted the analysis to patients who were prescribed zolpidem because there may be systematic differences between patients eligible to receive zolpidem and patients in whom zolpidem is not prescribed at all. Our institutional admission order sets provide physicians and other healthcare providers an option of selecting as‐needed zolpidem or trazodone as sleep aids. Zolpidem was the most common sleep aid that was prescribed to inpatients with a ratio of zolpidem to trazodone prescriptions being 2:1.
We used the pharmacy database to identify all eligible inpatients who were prescribed or administered either scheduled or as needed (PRN) zolpidem during the study period. All details regarding zolpidem prescription and administration were obtained from the inpatient pharmacy electronic database. This database includes all zolpidem orders that were placed in the inpatient setting. The database also includes details of dose and time of all zolpidem administrations.
The institution uses electronic medication profiles, and automated dispensing machines with patient profiles and point‐of‐care barcode scan technology, which forces highly accurate electronic documentation of the medication administered. The documentation of medication not given or patient refusal would be documented as not administered.
We reviewed the electronic medical record to ascertain demographics, as well as diagnoses of visual impairment, gait abnormality, cognitive impairment/dementia, insomnia, and delirium, based on International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes for these conditions (see Supporting Information, Appendix 1, in the online version of this article). These diagnostic codes were electronically abstracted from the medical record. The diagnosis codes are entered by medical coding specialists based on review of all provider notes. Hospital length of stay, Charlson comorbidity index scores, and Hendrich's fall risk scores from day of admission were abstracted from the individual electronic medical records. The nursing staff at our institution perform all the requisite assessments and electronically input all components required to calculate a Hendrich's fall risk score upon admission.
The Charlson index is a composite score calculated based on a patient's medical comorbidities. Each comorbidity is designated a score of 1, 2, 3, or 6 based on the risk of mortality associated with that condition.12 The Hendrich's fall risk is calculated based on the patient's current medication regimen, level of alertness, current medical condition, and the get up and go test.13A score of 5 or greater indicates increased risk of falling. These scores from the day of admission were available for all patients and were extracted from the nursing flow sheet.
At our institution, all falls are required to be called into a central event reporting system, and each fall receives an analysis regarding risk factors and proximal causes. We obtained details of all inpatient falls from this event system. The medication administration record, a part of the patient's electronic medical record, was accessed to identify all medications administered in the 24 hours prior to the fall. Medications were grouped into their respective pharmacologic classes. Antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioids were included in the analyses. These medications have previously been shown to be associated with increased risk of falls.14
Statistical analyses were performed using JMP (version 9.03, Cary, NC). Univariate analyses were performed to calculate the odds ratio of falling in inpatients who were administered zolpidem, in male patients, those admitted to a surgical floor, and in those that had a diagnosis of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, or delirium. Hospital length of stay, age, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores were treated as continuous variables, and odds ratio of falling per unit increase was calculated for each of these variables.
Multivariable logistic regression analysis was performed to calculate the odds of falling in patients who received zolpidem, after accounting for age, gender, insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, delirium, hospital length of stay, zolpidem dose, Charlson comorbidity index scores, and Hendrich's fall risk scores. Logistic regression analyses was repeated with only those factors that were significantly associated (P < 0.05) with falls or factors where the association was close to statistical significance (P < 0.08).
To account for the presence of other medications that might have increased fall risk, separate analyses using the MannWhitney U test comparing medication use in all hospitalized patients who sustained a fall were performed. We compared the rates of use of antidepressants, antipsychotics, antihistamines, sedative antidepressants (this class included trazodone and mirtazapine), benzodiazepines, and opioid medication in patients who were administered zolpidem to those patients not administered zolpidem in the 24 hours prior to sustaining a fall. This study had the requisite institutional review board approval.
RESULTS
There were 41,947 eligible admissions during the study period. Of these, a total of 16,320 (38.9%; mean age 54.7 18 years) patients were prescribed zolpidem. Among these patients, 4962 (30.4% of those prescribed, or 11.8% of all admissions) were administered zolpidem during the study period (Figure 1). The majority (88%) of zolpidem prescriptions were for PRN or as needed use. Patients who received zolpidem were older than those who were prescribed the medication but did not receive it (56.84 17.2 years vs 53.79 18.31 years; P < 0.001).

Patients who were prescribed and received zolpidem were more likely to be male, or have insomnia or delirium. They had higher Charlson comorbidity index scores and were more likely to be on a surgical floor. There was no statistically significant difference between patients who received zolpidem and patients who were prescribed but did not receive zolpidem in terms of their fall risk scores, length of hospital stay, rates of visual impairment, gait abnormalities, and cognitive impairment/dementia (all P > 0.05) (Table 1).
| Characteristics | Zolpidem Administered N = 4962 (%) | Zolpidem Not Administered N = 11,358 (%) | P Value |
|---|---|---|---|
| |||
| Age | 56.84 17.24 y | 53.8 18.30 y | <0.0001 |
| Males | 2442 (49.21) | 4490 (39.53) | <0.0001 |
| Falls | 151 (3.04) | 81 (0.71) | <0.0001 |
| Insomnia | 1595 (32.3) | 1942 (17.1) | <0.0001 |
| Delirium | 411 (8.28) | 378 (3.33) | <0.0001 |
| Cognitive impairment | 38 (0.77) | 63 (0.55) | 0.11 |
| Visual impairment | 84 (1.69) | 198 (1.74) | 0.82 |
| Gait abnormalities | 814 (16.40) | 1761 (15.50) | 0.15 |
| Patients on surgical floors | 2423 (48.8) | 5736 (50.50) | 0.05 |
| Length of hospital stay (mean/SD) | 4.26 8.03 d | 4.18 8.07 d | 0.60 |
| Charlson index (mean/SD) | 4.07 3.81 | 3.76 3.70 | <0.0001 |
| Hendrich's fall risk score (mean/SD) | 1.97 1.93 | 1.91 1.97 | 0.08 |
During the study period, there were a total of 672 total falls, with 609 unique patients falls (fall rate of 1.45/100 patients). Those who were administered zolpidem had an increased risk of falling compared to patients who were prescribed, but were not administered, zolpidem (fall rate of 3.04/100 patients vs 0.71/100 patients; odds ratio [OR] = 4.37, 95% confidence interval [CI] = 3.335.74; P < 0.001). Additionally, patients who received zolpidem had an increased risk of falling, as opposed to all other adult inpatients who did not receive zolpidemwhether prescribed zolpidem or not (3.04 falls/100 patients vs 1.24 falls/100 patients; OR = 2.50, 95% CI = 2.083.02; P < 0.001). The absolute increase in risk of sustaining a fall after receiving zolpidem as compared to all other adult inpatients was 1.8%, revealing a number needed to harm of 55.
During the study period, a total of 21,354 doses of zolpidem were administered, revealing a fall rate of 0.007 falls per dose of zolpidem administered (151/21,354). This was significantly greater than the baseline fall risk of 0.0028 falls per day of hospitalization (672/240,015 total hospital days) (P < 0.0001).
On univariate analyses, zolpidem use (OR = 4.37; 95% CI = 3.345.76; P < 0.001), male sex (OR = 1.36; 95% CI = 1.051.76; P = 0.02), insomnia (OR = 2.37; 95% CI = 1.813.08; P < 0.01), and delirium (OR = 4.96; 95% CI = 3.526.86; P < 0.001) were significantly associated with increased falls, as were increasing age, Charlson comorbidity index scores, fall risk scores, and dose of zolpidem (Table 2). While the association between the presence of cognitive impairment/dementia and falling was close to significant (OR = 2.89; 95% CI = 0.886.98; P = 0.075), the association between fall risk and the presence of visual impairment, gait abnormalities, and being on a surgical floor was not statistically significant.
| Risk Factor | Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 4.37 | 3.34 | 5.76 | <0.001 |
| Male sex | 1.36 | 1.05 | 1.76 | 0.02 |
| Insomnia | 2.37 | 1.81 | 3.08 | <0.001 |
| Delirium | 4.96 | 3.52 | 6.86 | <0.001 |
| Cognitive impairment | 2.89 | 0.88 | 6.98 | 0.075 |
| Visual impairment | 1.26 | 0.44 | 2.76 | 0.63 |
| Gait abnormalities | 1.22 | 0.86 | 1.68 | 0.26 |
| Being on a surgical floors | 0.88 | 0.68 | 1.15 | 0.36 |
| Age | 1.01 | 1.01 | 1.02 | <0.001 |
| Length of hospital stay | 0.99 | 0.98 | 1.01 | 0.93 |
| Charlson index | 1.29 | 1.26 | 1.32 | <0.001 |
| Hendrich's fall risk score∥ | 1.36 | 1.29 | 1.42 | <0.001 |
| Dose of zolpidem | 1.21 | 1.17 | 1.26 | <0.001 |
Zolpidem use continued to be significantly associated with increased fall risk (adjusted OR = 6.39; 95% CI = 3.0714.49; P < 0.001) after multivariable logistic regression analyses accounting for all factors where the association with increased fall risk was statistically significant or close to significant on univariate analyses (Table 3). On further analyses, of all adult non‐ICU, non‐pregnant inpatients who sustained a fall, those who sustained a fall after receiving zolpidem did not differ from other inpatients who did not sustain a fall in terms of their age (59.6 17.95 vs 63.2 16.8 years; P = 0.07), antidepressant (42.62% vs 39.70%; P = 0.39), antipsychotic (9.83% vs 13.78%; P = 0.24), antihistamine (6.55% vs 3.49%; P = 0.10), sedative antidepressant (14.75% vs 15.80%; P = 0.31), benzodiazepine (36.06% vs 26.86%; P = 0.83), or opioid use (55.73% vs 43.01%; P = 0.66).
| Characteristic | Adjusted Odds Ratio of Falling | Lower Confidence Interval* | Upper Confidence Interval* | P Value |
|---|---|---|---|---|
| ||||
| Zolpidem administration | 6.39 | 3.07 | 14.49 | <0.001 |
| Male sex | 1.24 | 0.93 | 1.67 | 0.14 |
| Insomnia | 1.60 | 1.17 | 2.17 | 0.003 |
| Delirium | 2.62 | 1.73 | 3.88 | <0.001 |
| Cognitive impairment | 1.47 | 0.33 | 4.53 | 0.56 |
| Age | 1.04 | 1.03 | 1.05 | <0.001 |
| Hendrich's fall risk score | 1.30 | 1.23 | 1.36 | <0.001 |
| Charlson index | 1.33 | 1.29 | 1.36 | <0.001 |
| Dose∥ | 0.94 | 0.82 | 1.06 | 0.37 |
DISCUSSION
In this study, zolpidem use was associated with an increased risk of falling in hospitalized patients. We calculate that for every 55 inpatients administered zolpidem, we might expect one more fall than would otherwise have occurred. To our knowledge, this is the largest study examining the association between zolpidem use and falls in an inpatient setting. Previous literature have not accounted for the presence of several other factors that could increase fall risk in hospitalized patients using zolpidem, such as visual impairment, gait abnormalities, and type of admission. In our study, insomnia and delirium were associated with higher rates of falls, however, the risk of sustaining a fall after receiving zolpidem continued to remain elevated even after accounting for these and multiple other risk factors.
Previous research in healthy volunteers found that subjects who received zolpidem experienced increased difficulty maintaining their balance.15, 16 The subject's ability to correct their balance, with their eyes closed and also with their eyes open, was adversely affected, indicating that both proprioception and visually enabled balance correction were impacted. Navigating obstacles in a hospital setting, where the patient is in a novel environment and on other medications that could impact balance, is potentially made significantly worse by zolpidem, thus resulting in an increased fall risk.
While a previous case‐control study of inpatients, 65 years and older, reported increased rates of zolpidem use among inpatients who sustained a fall, it did not report whether this association continued to remain significant after accounting for potential confounders.9 Another study, in a similar age group and carried out in an ambulatory community setting, found that patients who sustained a hip fracture were more likely to have received zolpidem in the 6 months prior to their fall.8 In this study, zolpidem use continued to be significantly associated with hip fractures after accounting for potential confounders such as the use of other medication, age, comorbidity index score, the number of hospital days, and the number of nursing days. Our study differs from these studies in that it was a cohort study in an inpatient setting, and we included all non‐pregnant adult hospitalized patients outside of the ICU. Also, we examined medication administration in the 24 hours prior to a fall rather than medications simply prescribed in the months prior to a fall.8 In our cohort of adult inpatients, the odds of zolpidem use among patients who fell was greater than those previously reported. This could indicate increased vulnerability in hospitalized patients compared to community‐dwelling elderly.
Insomnia, older age, and delirium have all been shown to be associated with an increased risk of falls in previous research.1517 In one study of community‐dwelling older adults, the authors found a higher risk of falling in subjects with insomnia, but not in those who received a hypnotic agent.15 Delirium increases the likelihood of nocturnal wandering, also associated with increased risk of fall. Our inpatient cohort study confirms these prior findings: insomnia, delirium, and older age were all associated with an increased risk of falling. However, zolpidem use continued to remain a significant risk factor for falls even after accounting for these risk factors.
Hospitalized patients are more likely to be physically compromised and on a greater number of medications compared to community‐dwelling subjects, and hence at increased risk of falling. Multiple classes of medications have been shown to be associated with an increased fall risk in hospitalized patients.14 In our study, the patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall in terms of their medication use. Zolpidem thus appears to increase the risk of falling beyond that attributable to other medications in hospitalized patients.
A recent United States Preventive Services Task Force on Prevention of Falls in Community Dwelling Older Adults recommendation indicates that withdrawal of medication alone does not appear to have a significant impact on fall rates.18 Another study indicates that reduced benozodiazepine use did not significantly reduce the rates of hip fractures in the community.19 While these studies indicate that fall risk is multifactorial and requires a complex set of interventions, our results indicate that there might be an association between zolpidem administration and falls in an inpatient setting. Changing order sets so that zolpidem use is not encouraged could potentially reduce fall rates in hospitalized patients, a step that we have already taken in our institution based upon these findings. Other potential measures to reduce fall risk include the use of fall precautions in patients who are prescribed zolpidem or use of non‐pharmacologic treatments for insomnia. However, these interventions would need to be empirically tested before they could be recommended with confidence.
The results of this study must be viewed in the light of some limitations. Although we included age, sex, zolpidem dose, length of hospital stay, Charlson comorbidity index score, fall risk score, and diagnoses of insomnia, visual impairment, gait abnormality, cognitive impairment/dementia, and delirium in our analyses, we were unable to account for the degree of severity of these conditions. There could also be other possible medical conditions that result in an increased risk of falling that were not accounted for in our analyses. While we did attempt to correct for insomnia and delirium diagnoses, transient complaints of insomnia or altered mental status may have been missed by our retrospective methodology, and perhaps could co‐associate with risk of falling. Furthermore, administration of zolpidem was associated with a higher risk of falls when compared to other patients who were prescribed zolpidem, and also when compared to all other patients regardless of zolpidem prescription. We used ICD‐9 codes to identify patients with insomnia, delirium, visual impairment, and gait abnormalities, and these could be prone to misclassification and possible ascertainment bias. Finally, we were unable to account for use of medications that might potentially increase the risk of falling in the entire cohort. We were, however, able to account for this in the subset of patients who sustained a fall, and did not note a difference between the group that received zolpidem and the group that did not. In these analyses, we were able to account for administration of these other medications, but not the dose or cumulative dose.
CONCLUSIONS
Our study, the largest in an inpatient cohort, reveals that zolpidem administration is associated with increased risk of falling even after accounting for insomnia, delirium, and multiple other risk factors. Patients who sustained a fall after receiving zolpidem did not differ from other patients who sustained a fall, in terms of age or use of other medications conferring increased fall risk. Although insomnia and delirium are also associated with an increased risk of falling, addition of zolpidem in this situation appears to result in a further increase in fall risk. Presently, because there is limited evidence to recommend other hypnotic agents as safer alternatives in inpatient settings, non‐pharmacological measures to improve the sleep of hospitalized patients should be investigated as preferred methods to provide safe relief from complaints of disturbed sleep.
Acknowledgements
The authors acknowledge Anna Halverson, RN, from Nursing Practice Resources, for providing patient fall data from the Mayo Clinic Rochester Event Tracking System used in analysis; and Erek Lam, MD, for helping with data abstraction from the electronic medical record.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
- ,,,,.Noise and sleep among adult medical inpatients: far from a quiet night.Arch Intern Med.2012;172:68–70.
- ,.Sleep disruption experienced by surgical patients in an acute hospital.Br J Nurs.2008;17(12):766–771.
- .Sleep disruption in hospitalized adults.Medsurg Nurs.2008;17:391–395.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Ten‐year trends in the pharmacological treatment of insomnia.Sleep.1999;22:371–375.
- ,,.Hypnotics' association with mortality or cancer: a matched cohort study.BMJ Open2012:2:e000850–e000850.
- ,,,.Effect of hypnotic drugs on body balance and standing steadiness.Sleep Med Rev.2010;14:259–267.
- ,,,,.Zolpidem use and hip fractures in older people.J Am Geriatr Soc.2001;49:1685–1690.
- ,,, et al.Medical conditions and medications as risk factors of falls in the inpatient older people: a case‐control study.Int J Geriatr Psychiatry2011;26:602–607.
- Department of Health and Human Services Partnership for Patients.2012. Available at: http://innovation.cms.gov/initiatives/partnership‐for‐patients/index.html. Accessed on July 1, 2012.
- ,,.Meta‐analysis: multidisciplinary fall prevention strategies in the acute care inpatient population.J Hosp Med.2012;7(6):497–503.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Hospital falls: development of a predictive model for clinical practice.Appl Nurs Res.1995;8:129–139.
- ,,, et al.Meta‐analysis of the impact of 9 medication classes on falls in elderly persons.Arch Intern Med.2009;169:1952–1960.
- ,,, et al.Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes.J Am Geriatr Soc.2005;53:955–962.
- ,.Fall events in geriatric hospital in‐patients. Results of prospective recording over a 3 year period [in German].Z Gerontol Geriatr2004;37:9–14.
- ,,,.Serious falls in hospitalized patients: correlates and resource utilization.Am J Med.1995;99;137–143.
- United States Preventive Services Task Force on the Prevention of Falls in Community‐Dwelling Older Adults.2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfalls.htm. Accessed on July 1, 2012.
- ,,, et al.Effect of New York State regulatory action on benzodiazepine prescribing and hip fracture rates.Ann Intern Med.2007;146;96–103.
Copyright © 2012 Society of Hospital Medicine