User login
Longitudinal Dynamic in Weight Loss Impacts Clinical Outcomes for Veterans Undergoing Curative Surgery for Colorectal Cancer
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
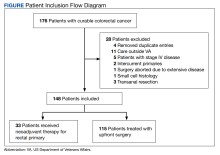
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
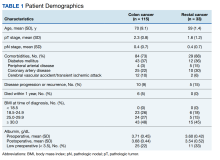
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
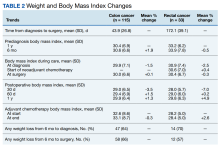
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
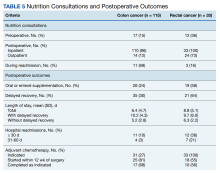
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
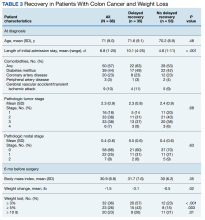
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285