User login
Longitudinal Dynamic in Weight Loss Impacts Clinical Outcomes for Veterans Undergoing Curative Surgery for Colorectal Cancer
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
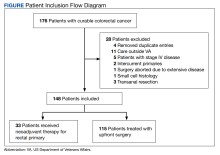
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
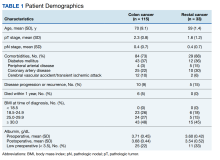
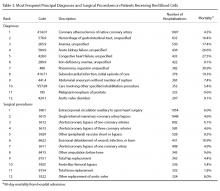
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
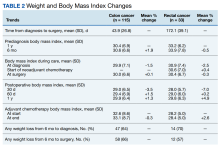
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
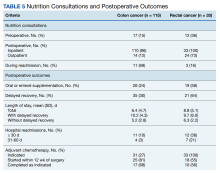
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
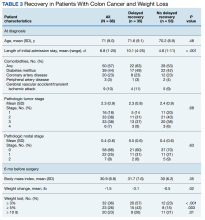
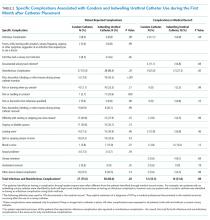
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
Improving Hand Hygiene Adherence in Healthcare Workers Before Patient Contact: A Multimodal Intervention in Four Tertiary Care Hospitals in Japan
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
© 2020 Society of Hospital Medicine
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine
A Longitudinal Study of Transfusion Utilization in Hospitalized Veterans
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Assessing Vascular Nursing Experience
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
© 2015 Society of Hospital Medicine