User login
Urothelial Carcinoma: Muscle-Invasive and Metastatic Disease
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
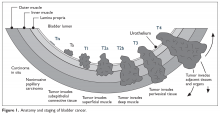
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
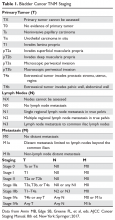
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
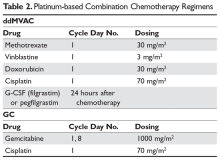
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
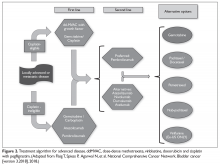
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
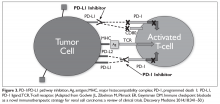
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.