User login
Stress‐Ulcer Prophylaxis for General Medical Patients
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
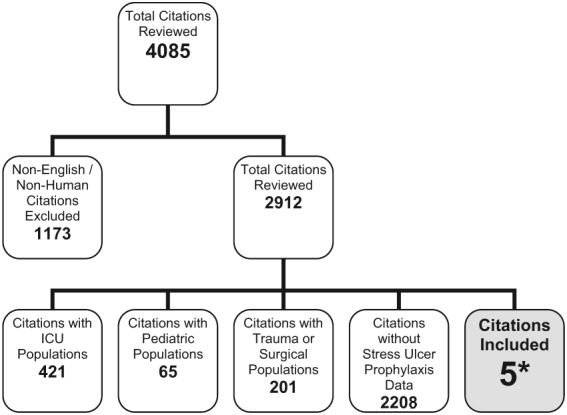
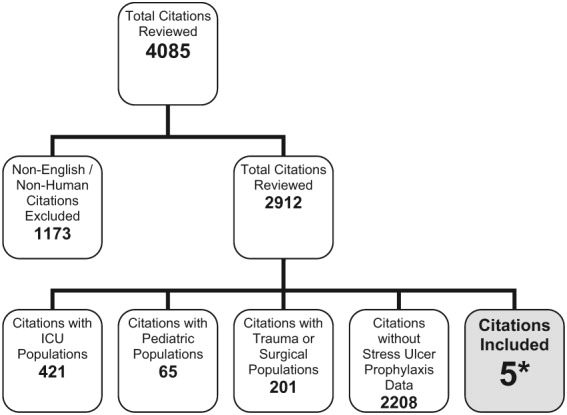
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).
Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).

Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
Patients suffering from a critical life‐threatening illness have long been known to have an increased risk of spontaneous upper gastrointestinal bleeding, even in the absence of previously known gastrointestinal pathology. This phenomenon is generally known as the stress‐ulcer syndrome or stress‐related mucosal disease. Although the incidence in critically ill patients has declined in recent years to less than 10%, the mortality rate in patients experiencing bleeding is often considered to be nearly 50%.1, 2 Various pathophysiological processes including respiratory failure, sepsis, coagulopathy, burns, and severe trauma have been implicated in the development of stress ulcers in critically ill patients.1, 3 The administration of acid‐suppressing medications such as histamine‐2 receptor antagonists, proton‐pump inhibitors, and sucralfate has been shown to decrease the risk of stress‐related gastrointestinal bleeding in these patients.46 As a result, it is standard practice in many intensive care units to use such medications, commonly referred to as stress‐ulcer prophylaxis, to reduce the production of gastric acid and raise intragastric pH. Many intensivists prescribe stress‐ulcer prophylaxis to all ICU patients, including those without risk factors.7
Patients admitted to general medical wards also experience gastrointestinal bleeding. There appears to be an association between stress‐ulcer bleeding in general medical patients and overall severity of illness, similar to that in critically ill patients. Risk factors may include ischemic heart disease, chronic renal failure, and a prior intensive care unit stay or mechanical ventilation.8, 9 Studies in limited populations found that 3% of patients admitted with acute stroke and 13% of patients admitted with renal failure have experienced bleeding. However, only about half these episodes were clinically significant.10, 11 A more recent review that included a much larger and medically diverse population found a rate of less than 1%. In this study mortality did not differ between patients with and without bleeding.12 An older report found that mortality in a set of 125 hospitalized patients with secondary gastrointestinal bleeding was 28%, but only a small fraction of the deaths was directly attributable to the bleeding episode.13
Widespread use of acid‐suppressive therapy for stress‐ulcer prophylaxis in general medical settings has been recognized, especially among patients cared for by medical residents. However, this practice has been significantly less well characterized than the use of stress‐ulcer prophylaxis in critical care settings.1416 This article reports a systematic review of the literature to answer 2 questions: (1) What is the frequency of prescription of acid‐suppressive therapy for stress‐ulcer prophylaxis among adult general medical inpatients? (2) What evidence exists to support this practice?
METHODS
Data Sources
This review was designed and conducted using the principles of systematic reviews set forth by Cook, Counsell, and Meade and reported elsewhere.1719 The MEDLINE database (from 1966 to October 2005) and the Cochrane Central Register of Controlled Trials (fourth quarter 2005) were searched using the following medical subject heading search terms: stress ulcer, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding and prophylaxis, gastrointestinal hemorrhage/peptic ulcer hemorrhage/gastrointestinal bleeding, and hospital, and stress‐related mucosal disease. The retrieved articles were then limited to those written in English that involved human subjects. The titles and abstracts of all articles were individually reviewed, and the full text of any potentially relevant article was obtained and evaluated for inclusion. The bibliographies of studies chosen for inclusion were also reviewed.
Study Selection
Studies were chosen for entry if they contained significant data about either of the 2 objectives of this review: (1) the frequency of use of stress‐ulcer prophylaxis in general medical patients and (2) gastrointestinal bleeding outcomes in patients prescribed such prophylaxis. Articles that focused primarily or exclusively on surgical, trauma, pediatric, or nonhospitalized medical patients, as well as those that clearly stated that the subjects were drawn from an intensive care unit setting, were excluded. For this purpose, studies focusing primarily on patients on mechanical ventilation were assumed to be referencing an intensive care unit population and were excluded.
Articles chosen to fulfill the first objective were required to contain information on prophylaxis use in a diverse medical population. Studies that did not clearly delineate the indications for acid‐suppressive therapy were excluded. Those chosen to fulfill the second objective were excluded if acid‐suppressive therapy was prescribed for an indication other than stress‐ulcer prophylaxis. This would include the treatment of any other gastrointestinal pathology, including gastrointestinal bleeding present on admission to the hospital. Finally, articles chosen for the second objective were required to be randomized and controlled.
Study Evaluation
The controlled trials selected for review were examined according to the methodology in the CONSORT statement, as reported elsewhere, and its subsequent revision.20, 21 The primary author exclusively determined which articles met inclusion criteria.
RESULTS
The search criteria identified 3979 citations from the electronic databases and 106 references from the included studies. After eliminating non‐English‐language articles and articles that did not have human subjects, 2912 articles were examined. Of these, only 5 citations met the inclusion criteria (Fig. 1).

Frequency of Use of Stress‐Ulcer Prophylaxis in General Medical Patients:
Three descriptive reports addressed this issue. The first, by Nardino et al., examined all patients admitted to a general medical ward of a community teaching hospital over a 3‐month period.22 Of the 226 patients studied, 122 (54%) received some form of acid‐suppressive therapy, with 47 (21% of the total) receiving therapy as either stress‐ulcer prophylaxis or for no specific indication. Most of these (62%) received H2 receptor antagonists. The most frequent indication, reported in 33 patients (15% of the total population), was stress‐ulcer prophylaxis in patients believed to be at low risk of bleeding. In an additional 12 patients (5%), no clear rationale for use could be discerned from the medical record. The authors believed that 2 patients who received acid‐suppressive therapy as prophylaxis were at sufficiently high risk to justify such use because of previous prolonged mechanical ventilation.
The second report by Parente et al. studied all patients admitted to a general medical and surgical ward over a 1‐month period.23 Of the 799 patients reviewed, 71% were admitted to the medical or neurology service. Acid‐suppressive therapy was prescribed to 374, with 246 receiving therapy either as prophylaxis for stress ulcer or for no indication (47% and 31% of the total population, respectively). Proton‐pump inhibitors were the most commonly used drugs. Again, the most common indication was stress‐ulcer prophylaxis in low‐risk patients, which occurred in 177 (22% of the total population). An additional 22 patients (3%) had no clearly documented indication. Forty‐seven patients (6%) were judged by the authors to warrant stress‐ulcer prophylaxis based on risk of bleeding. Data specific to the medical service was not reported.
Finally, Gulotta et al. examined the records of 3685 inpatients at 20 hospitals on a randomly selected day. Of these inpatients, 1758 were admitted to an internal medicine service.24 There were 987 patients (28.6%) from the total population and 396 (22.5%) from those admitted to the medical services treated with an acid‐suppressive agent. Prevention of stress ulceration was the documented indication for 205 (21% of patients prescribed acid‐suppressive therapy and 6% of the total population), but the authors did not provide specific data for the medical service (Table 1). Unfortunately, as all these studies were cross‐sectional, no subsequent information on bleeding outcomes was provided.
| |||
| Author, year of publication, reference | Nardino, 200021 | Parente, 200322 | Gullota, 199723 |
| Total population | 226 | 799 | 3685 |
| Admitted to medical service (%) | 226 (100) | 568 (71) | 1758 (48) |
| Receiving acid suppression for any indication (%) | 122 (54) | 374 (47)* | 987 (29)* |
| Receiving acid suppression as stress‐ulcer prophylaxis: all risk groups, including patients with no clear indication for use (%) | 47 (21) | 246 (31) | 205 (6) |
| Acid suppression as stress ulcer prophylaxis: high risk patients (%) | 2 (0.9) | 47 (6) | NR |
| Acid suppression as stress‐ulcer prophylaxis: low risk patients and those without a clear indication (%) | 45 (20) | 199 (25) | NR |
Gastrointestinal Bleeding Outcomes in Patients on Prophylaxis
Only 2 trials sufficiently met the inclusion criteria and were included for review. The first trial, by Estruch et al., was a placebo‐controlled, randomized trial of magaldrate for gastrointestinal bleeding prophylaxis.25 Magaldrate is an aluminum and magnesium containing antacid sold under various trade names. One hundred patients admitted to a general hospital ward were studied. These patients were consecutive admissions with presumed risk factors for stress‐ulcer disease. Risk factors were defined as respiratory failure with a PO2 less than 60 (not requiring intubation), heart failure requiring inotropic support, sepsis, stroke, hepatic encephalopathy or jaundice, renal failure, hypotension, previous gastrointestinal disease, treatment with corticosteroids (more than 250 mg of prednisone per day), nonsteroidal anti‐inflammatories, heparin, or warfarin. Patients with recognized gastrointestinal bleeding, including occult blood in the stool at study entry and those who were on an outpatient acid‐suppressive regimen were excluded. A total of 52 patients were randomized to magaldrate, 800 mg 5 times per day, and 48 to placebo. Gastrointestinal bleeding was defined broadly to include patients with overt bleeding as well as those with only occult blood in the stool.
The intervention and placebo groups were well matched by age, previous history of peptic ulcer or gastritis, and previous use of corticosteroids, NSAIDs, or warfarin. There were significantly more men in the placebo group (69% vs. 46%). The patients were examined daily for evidence of gastrointestinal bleeding including occult blood in the stool. One patient (1.9%) receiving magaldrate and 11 patients (22.9%) receiving placebo had evidence of gastrointestinal bleeding (P < .01, ARR = 21%, NNT = 5). The lone patient in the magaldrate group who experienced bleeding was found to have only occult blood in the stool and experienced a drop in hematocrit of 2%. Three of the patients in the placebo group who bled presented with frank melena, whereas the rest were found to have occult blood in the stool. Endoscopic examination showed an ulcer in 2 patients and erosive gastritis in eight. Three of these bleeding episodes were clinically significant (6% of the placebo group), as shown by a drop in hematocrit of more than 10% and a requirement for transfusion of 2 or more units of blood. The authors did not state whether these clinically significant bleeds presented first with melena or only occult blood in the stool.
One patient in the placebo group died, which was a result of a hemorrhagic stroke, and 2 patients in the magaldrate group died, both due to malignancy. The investigators did not attribute any of these deaths to the intervention studied or to gastrointestinal causes. Side effects were minimal in both groups, and no patient discontinued therapy prematurely. A subgroup analysis was performed comparing rates of bleeding between groups based on number of presumed risk factors. There was no significant difference in bleeding between the magaldrate and placebo group for patients with only 1 risk factor, but there was a significant absolute risk reduction of 20.8% for prophylaxis when the patient had 2 risk factors and a 35.4% absolute risk reduction when the patient had 3 or more risk factors. This corresponds to a NNT of only 3 for these more seriously ill patients. Both of these were statistically significant (Table 2). Based on this analysis, the authors concluded that seriously ill general ward patients had a relatively high rate of stress‐ulcer bleeding and therefore should receive stress‐ulcer prophylaxis.
| Magaldrate | Placebo | |
|---|---|---|
| ||
| Patients enrolled | 52 | 48 |
| Age (SD) | 64.5 (16.8) | 67.4 (16.1) |
| Men (%) | 24 (46) | 33 (69) |
| Average days in study | 6.78 | 7.34 |
| Deaths | 2 | 1 |
| Bleeding episodes | ||
| Total (AR), P < 0.01 | 1 (1.9) | 11 (22.9) |
| Severe* (AR), P = NR | 0 (0) | 3 (6.3) |
| ARR for any bleeding (NNT) | 21 (5) | N/A |
| Episodes of bleeding per number of risk factors | ||
| 1 (AR), P = NS | 0/12 (0) | 1/11 (9.1) |
| 2 (AR), P = 0.02 | 0/24 (0) | 5/24 (20.8) |
| 3 (AR), P = 0.03 | 1/16 (6.2) | 5/12 (41.6) |
| ARR for any bleeding in patients with 3 risk factors (NNT) | 35.4 (3) | N/A |
The second trial, by Grau et al., was conducted in the same hospital as the previous investigation.26 Over a 10‐month period, the authors evaluated consecutive patients admitted to a general hospital ward with the same risk factors as in the previous study. Patients with respiratory failure, heart failure, sepsis, stroke, liver or kidney failure, or who were being treated with corticosteroids, heparin, or warfarin were included. Eligible patients were randomized to a single nightly dose of cimetidine 800 mg or sucralfate 1 g every 6 hours. Again, patients with evidence of gastrointestinal bleeding on admission or outpatient use of acid suppressants were excluded. These authors also broadly defined gastrointestinal bleeding to include symptomatic patients as well as those who developed occult blood in the stool during the index admission.
A total of 144 patients met inclusion criteria and were randomized, 74 to cimetidine and 70 to sucralfate. Both groups were well matched in age and length of hospital stay, but there were more men in the cimetidine group (66% vs. 53%), and more patients in the cimetidine group (16 vs. 7) were readmitted to the hospital during the study period. None of these readmissions were attributed to gastrointestinal bleeding. Again, the patients were examined daily for overt bleeding as well as for occult blood in the stool. Two patients in each group bled during the study. In both patients in the cimetidine group, bleeding was detected by stool occult blood testing and was not clinically significant. Endoscopy was normal in 1 patient and showed mild gastritis in the other; neither patient required transfusion. The bleeding in the patients in the sucralfate group was more severe and presented with melena and coffee‐ground emesis. Endoscopic examination found erosive esophagitis in 1 and a duodenal ulcer in the other; both required transfusion. Therefore, the rate of clinically significant bleeding was 2.9% in the sucralfate group and 0 in the cimetidine group. Although all patients were considered at risk of bleeding because of inclusion criteria, a subgroup analysis failed to find any significant difference in risk factors between patients who bled and those who did not.
During the study, 3 patients in the cimetidine group and 2 in the sucralfate group died. The causes were cardiac failure, sepsis, pulmonary embolism, and malignancy. The authors did not attribute any of these deaths to gastrointestinal bleeding or the studied intervention and they were excluded from the final analysis. Side effects in both groups were mild and did not lead to discontinuation in any patient.
The authors concluded that the overall rate of bleeding episodes in this investigation was similar to that of the patients treated with magaldrate in the previous study (approximately 3%), and therefore, seriously ill patients admitted to general medical wards benefit from stress‐ulcer prophylaxis. However, there was no evidence to recommend a specific class of medication for this purpose (Table 3).
| Cimetidine | Sucralfate | |
|---|---|---|
| ||
| Patients enrolled | 74 | 70 |
| Age (SD) | 67 (12) | 64 (13) |
| Men (%) | 47 (66) | 36 (53) |
| Days in study | 8.8 | 8.7 |
| Readmissions (P < 0.05) | 16 | 7 |
| Deaths (P = NS) | 3 | 2 |
| Number included for analysis | 71 | 68 |
| Bleeding episodes | ||
| Total (AR), P = NR | 2 (2.7) | 2 (2.9) |
| Severe* (AR), P = NR | 0 | 2 (2.9) |
DISCUSSION
To our knowledge, this is the first systematic review of the literature that examined the use of acid‐suppressing medications as stress‐ulcer bleeding prophylaxis among general medical patients. Results indicate that there is widespread use of these medications among general medical patients, but little evidence demonstrating a reduction in clinically important gastrointestinal bleeding.
Nardino et al., Parente et al., and Gullota et al. indicated that acid‐suppressive therapies are prescribed to 29%‐54% of hospitalized inpatients. The most common indication for such therapy is stress‐ulcer prophylaxis in patients believed to be at low or no risk, which was true for 20%‐25% of all such patients. Interestingly, both Nardino et al. and Parente et al. assumed there were risk factors that place some general medical patients in a higher‐risk category. In their assessment, these patients warrant prophylaxis. This is somewhat problematic though, as such risk factors have yet to be firmly established. All studies were localized, and results should be confirmed in a larger series that spans multiple institutions. Widespread use of stress‐ulcer prophylaxis may be driven by fear of the previously reported high mortality rates associated with stress‐ulcer bleeding. This fear may be largely unjustified, as overall rates of bleeding episodes appear low.12 Furthermore, patients who die with stress‐ulcer‐related bleeding likely die from their underlying severe illness rather than the bleed itself.
We identified only 2 studies that tested the effectiveness of stress‐ulcer prophylaxis in general medical populations. Both indicated a relatively low risk of gastrointestinal bleeding in patients receiving prophylaxis. Most notably, the work by Estruch et al. comparing an antacid regimen (magaldrate) to placebo showed a significant reduction in bleeding in the active treatment group. However, these trials possess characteristics that limit their applicability to a broad medical population. In particular, these trials were designed to represent only patients with severe illness, many of whom possessed presumed risk factors for stress‐ulcer bleeding. Although all the patients in these 2 series were managed on a general medical ward, many (eg, heart failure patients requiring inotropic support) would likely qualify for intensive care at some institutions. The rate of minor gastrointestinal hemorrhages in the placebo group of the magaldrate trial was significantly higher than in previous observational trials, further suggesting that this population had greater severity of illness than a typical medical population. In addition, although the studies contributed some useful information about severely ill patients, both controlled trials had design limitations. Neither study described why the respective populations were chosen or how the sample sizes were derived. More important, neither was double‐blinded. In sum, given the small number of trials, the limited generalizability to more severely ill patients, and design limitations, the existing literature provides minimal guidance about stress‐ulcer prophylaxis in a diverse inpatient service.
There were no major drug‐related adverse effects reported in these trials, and all the acid‐suppressive drugs currently available are considered relatively safe. However, widespread prophylaxis could result in adverse outcomes on balance. For example, in intensive care populations, there is evidence of an increased risk of nosocomial pneumonia associated with universal acid suppression.27 Many of these patients, however, have other risk factors for pneumonia such as mechanical ventilation.28, 29 Similarly, there has been an association between proton‐pump inhibitor use and increased risk for Clostridium difficileassociated diarrhea.30, 31 Also, H2 receptor antagonists have been implicated in thrombocytopenia, but this is still somewhat controversial.32 Whether these or any other adverse events occur commonly in general medical patients is unclear. Finally, every medication prescribed to inpatients increases the cost of the hospitalization and places a further strain on the financial resources of many already troubled health care delivery systems. For example, a 1997 study found that the use of ranitidine for stress‐ulcer prophylaxis cost $84.81 per day and omeprazole cost $39.52 per day, and those costs would presumably be higher today.33 These costs increase more if patients are continued on such medications after discharge. Clinicians have an obligation to ensure that the therapies they prescribe do not result in increased cost or harm, unless there is at least a reasonable expectation for average net benefit. More information is needed to guide such judgments for stress‐ulcer prophylaxis in non‐ICU patients.
As with all reviews, this one had some limitations. Although we searched a wide body of medical literature, some relevant work may not have been considered. Any published work not indexed by the Medline database or not listed in the Cochrane database of controlled trials would not have been part of this review. In addition, articles written in a language other than English and unpublished works were not examined. Therefore, it is possible that others have investigated this topic and collected information that would alter our results. However, this seems unlikely given the paucity of relevant studies in the wide body of literature that was examined. Finally, the primary author was exclusively responsible for identifying which studies met the inclusion criteria. It is conceivable that additional reviewers would have considered other studies to be relevant to the analysis.
Because stress‐ulcer prophylaxis appears to be widely used in patients hospitalized outside the intensive care unit, it is necessary to determine the efficacy and safety of this practice. Unfortunately, research in this area is sparse. The only 2 trials evaluating this topic, although suggesting a benefit for prophylaxis in selected higher‐risk populations, did not provide guidance for prophylaxis among a broad population of hospitalized medical patients. The present body of evidence does not clearly support or refute the use of stress‐ulcer prophylaxis in a general medical population. An appropriately powered randomized, controlled trial in a diverse population of general medical patients would clarify this issue.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients.Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients with and without stress‐ulcer prophylaxis.Intensive Care Med.2003;29:1306–1313.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,.A prospective study of omeprazole suspension to prevent clinically significant gastrointestinal bleeding from stress ulcers in mechanically ventilated trauma patients.J Trauma.1998;44:527–533.
- ,,,.Stress ulcer prophylaxis in patients on ventilator.Trop Gastroenterol.2003;24:124–128.
- ,,,.Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32:2008–2013.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case‐control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,,,.Upper gastrointestinal hemorrhage. Comparison of the causes and prognosis in primary and secondary bleeders.Scand J Gastroenterol.1994;29:795–798.
- ,,.Gastrointestinal hemorrhage after acute stroke.Stroke.1996;27:421–424.
- ,,,,,.Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure.Kidney Int.2001;59:1510–1519.
- ,,.Hospital‐acquired gastrointestinal bleeding outside the critical care unit.J Hosp Med.2006:1:13–20.
- ,,,,.Predictors of mortality in hospitalized patients with secondary upper gastrointestinal haemorrhage.J Intern Med.1995;237:331–337.
- ,,,,,.Overuse of acid suppressive therapy in hospitalised patients with pulmonary diseases.Respir Med.2003;97:1143–1150.
- ,,.Drug usage evaluation: H2‐receptor antagonist use in 30 hospitals.Hosp Formul.1991;26:725–736
- ,.Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents: A practice‐based educational intervention.J Gen Intern Med.2006:21:498–500.
- ,,.Systematic reviews: synthesis of best evidence for clinical decisions.Ann Intern Med.1997;126:376–380.
- .Formulating questions and locating primary studies for inclusion in systematic reviews.Ann Intern Med.1997;127:380–387.
- ,.Selecting and appraising studies for a systematic review.Ann Intern Med.1997;127:531–537.
- ,,, et al.Improving the quality of reporting of randomized controlled trials. The CONSORT statement.JAMA.1996;276:637–639.
- ,,.The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials.JAMA.2001;285:1987–1991.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–3122.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29:325–329.
- ,,, et al.Prophylaxis of gastrointestinal tract bleeding with magaldrate in patients admitted to a general hospital ward.Scand J Gastroenterol.1991;26:819–826.
- ,,,,.Prophylaxis of gastrointestinal tract bleeding in patients admitted to a general hospital ward. Comparative study of sucralfate and cimetidine.Scand J Gastroenterol.1993;28:244–248.
- ,,, et al.Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta‐analyses.JAMA.1996;275:308–314.
- ,,.Nosocomial infections and risk factors in intensive care units.New Microbiol.2003;26:299–303.
- ,,.A predictive risk index for nosocomial pneumonia in the intensive care unit.Am J Med.1992;93:135–142.
- ,,, et. al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004:171:33–38.
- ,,,.Use of gastric acid‐suppressive agents and the risk of community‐acquired clostridium difficile‐associated disease.JAMA.2005:294:2989–2995.
- ,,,.H‐2 antagonist‐induced thrombocytopenia: is this a real phenomenon?Intensive Care Med.2002;28:459–465.
- ,,,.Comparison of omeprazole and ranitidine for stress ulcer prophylaxis.Dig Dis Sci.1997:42:1255–1259.