Article
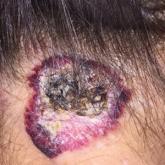
Large Hemorrhagic Plaque With Central Crusting
- Author:
- Caitlin Farmer, MD
- Devika Patel, MD
- Jason D. Pimentel, MD
- Tor Shwayder, MD
A 54-year-old woman presented with a large hemorrhagic plaque on the left side of the posterior neck with central brown-yellow crusting and a few...
Article
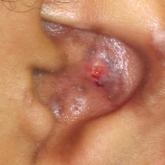
Gottron Papules Mimicking Dermatomyositis: An Unusual Manifestation of Systemic Lupus Erythematosus
- Author:
- Ji Won Ahn, MD
- Sherry Yang, MD
- Katherine Johnson, DO
- Tor Shwayder, MD
