User login
Comparison of Renal Function Between Tenofovir Disoproxil Fumarate and Other Nucleos(t)ide Reverse Transcriptase Inhibitors in Patients With Hepatitis B Virus Infection
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
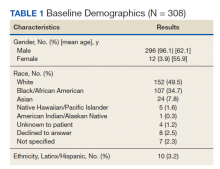
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
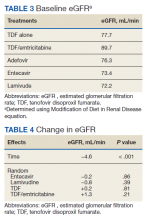
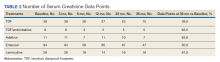
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Community-Acquired Pneumonia: Treatment
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
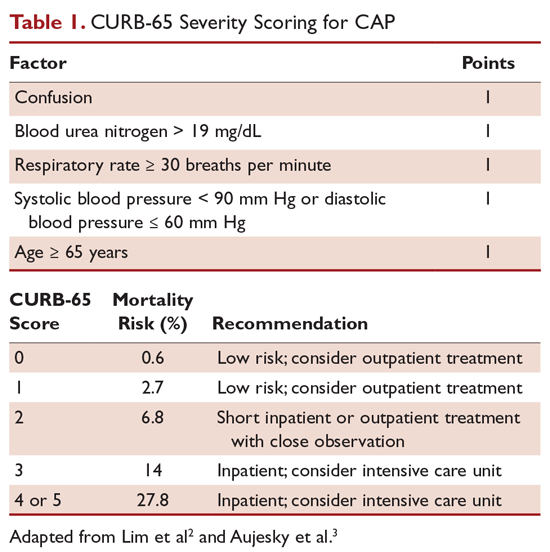
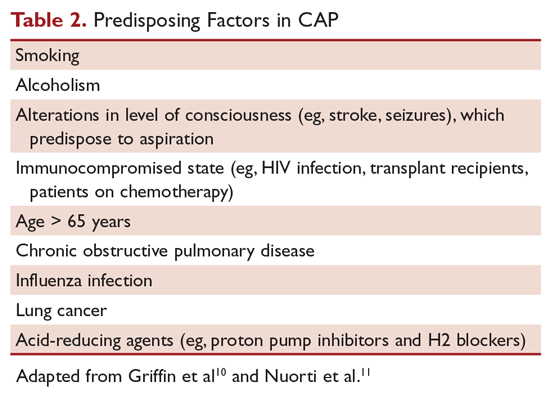
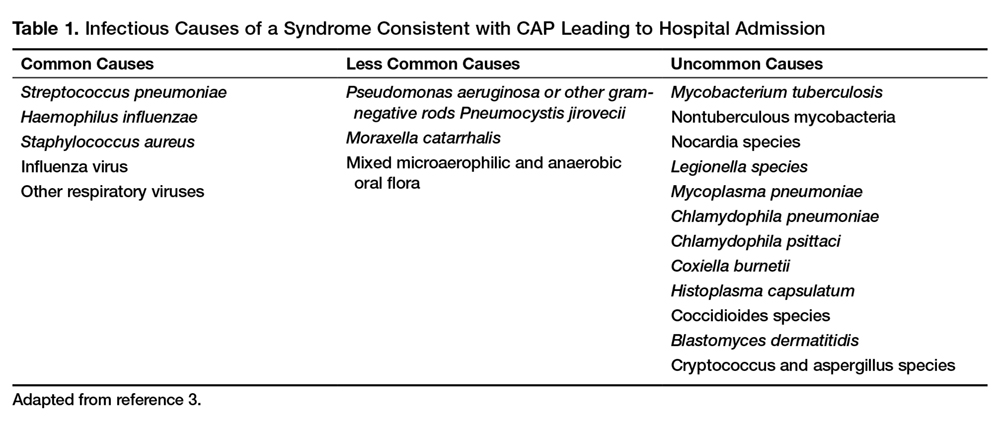
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
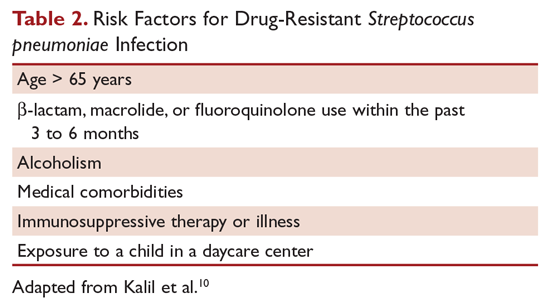
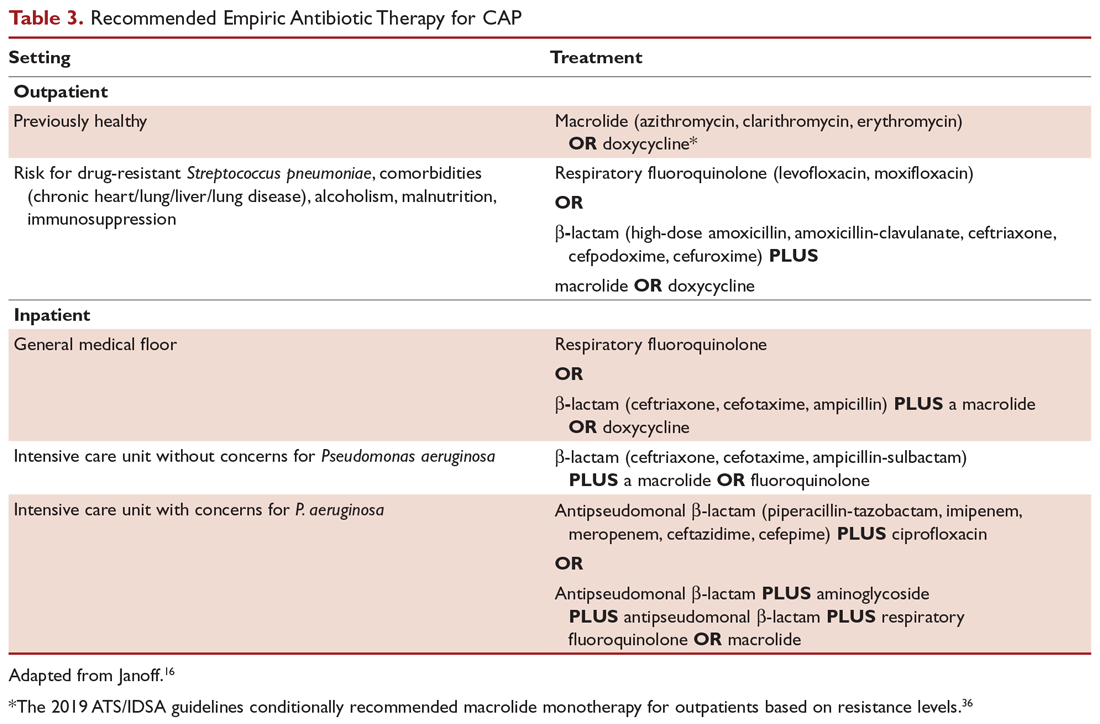
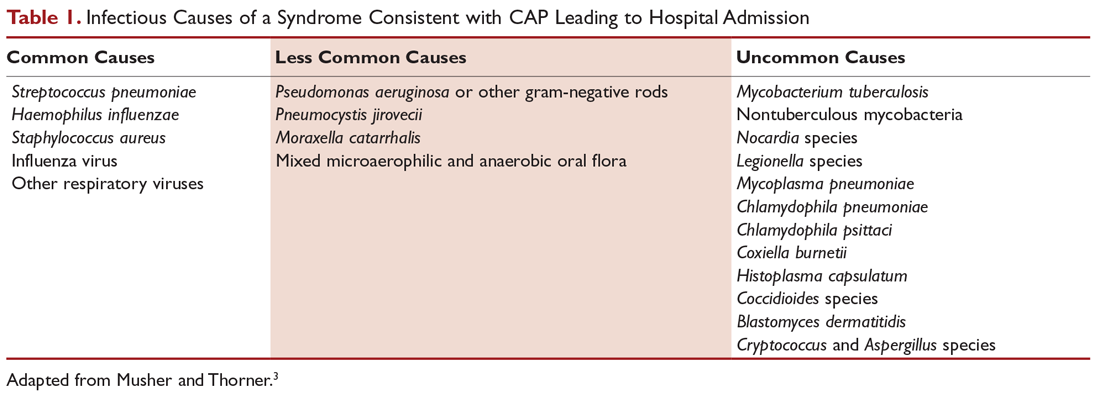
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
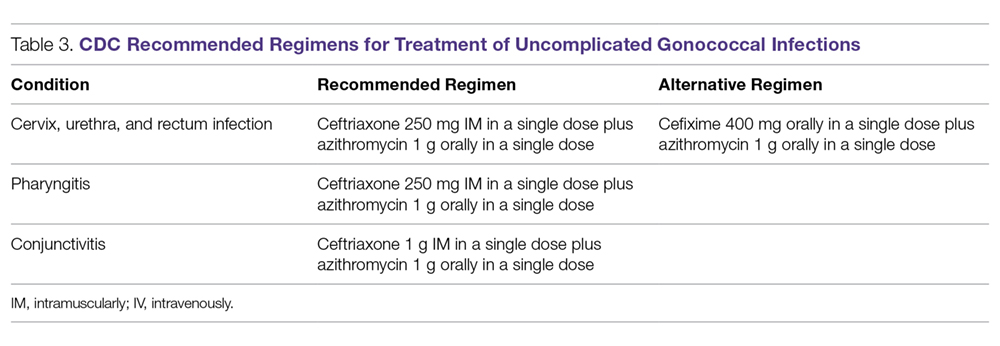
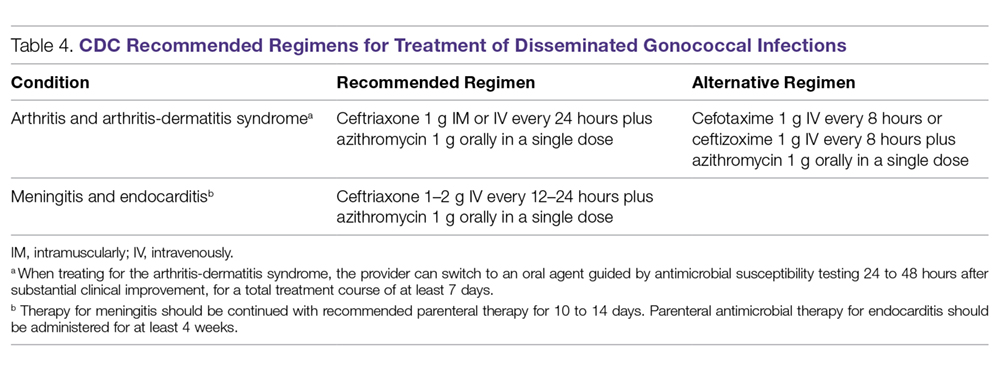
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
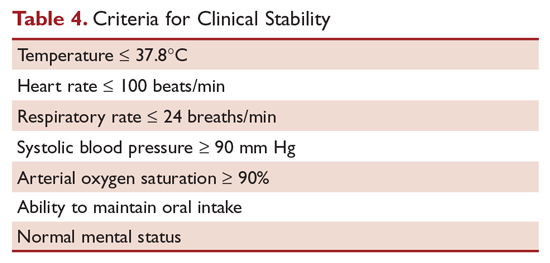
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
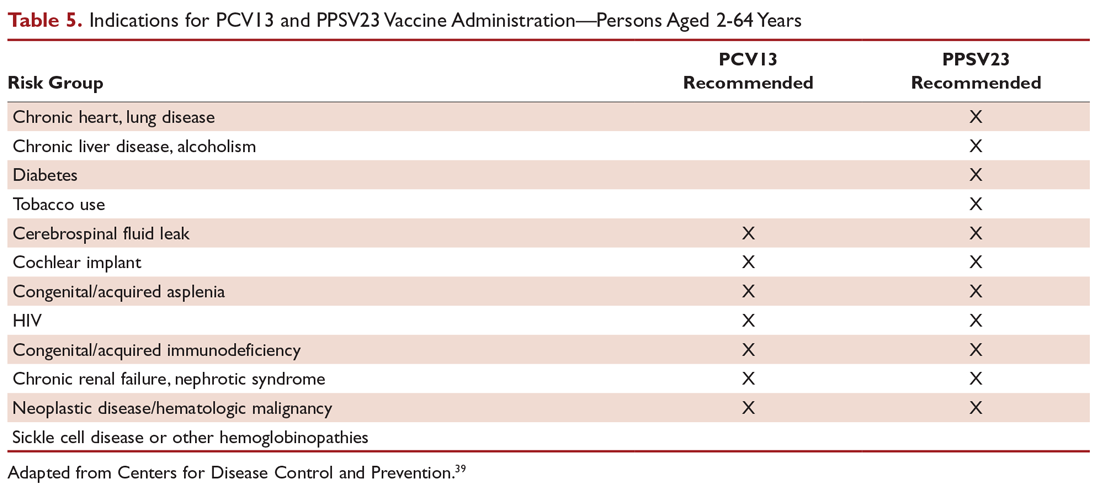
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Community-Acquired Pneumonia: Evaluation and Diagnosis
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Management of Community-Acquired Pneumonia in Adults
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.