User login
Comparison of Renal Function Between Tenofovir Disoproxil Fumarate and Other Nucleos(t)ide Reverse Transcriptase Inhibitors in Patients With Hepatitis B Virus Infection
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
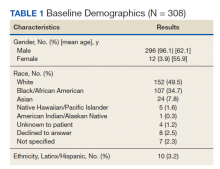
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
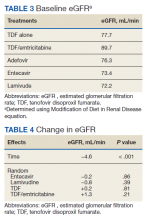
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
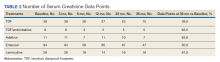
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
“I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency room
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.