User login
Adipose Flap Versus Fascial Sling for Anterior Subcutaneous Transposition of the Ulnar Nerve
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
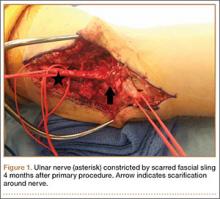
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
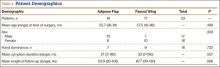
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
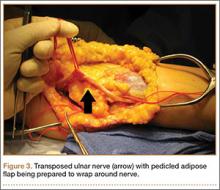
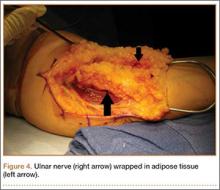
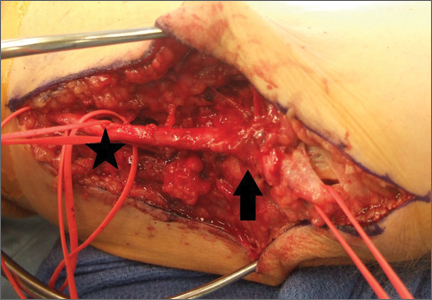
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.