User login
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
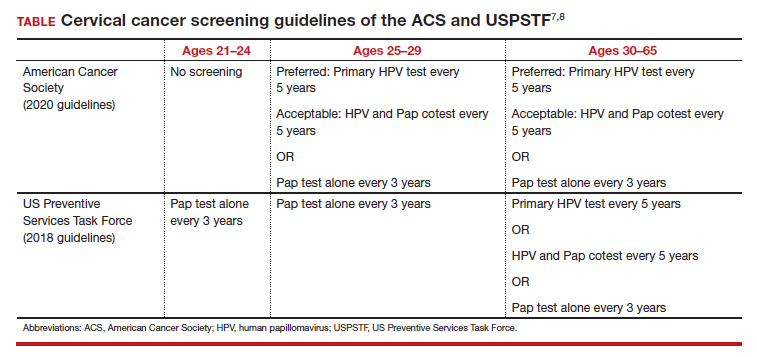
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22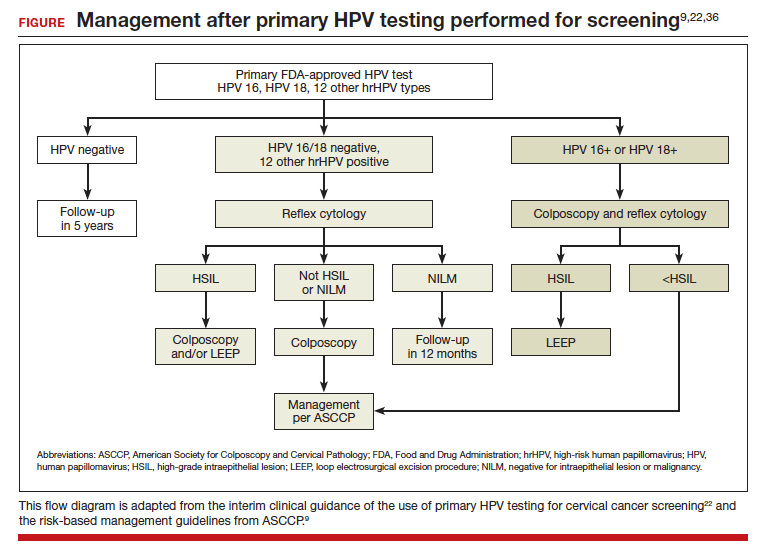
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.

