User login
Is the 9-valent HPV vaccine safe and effective long term?
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
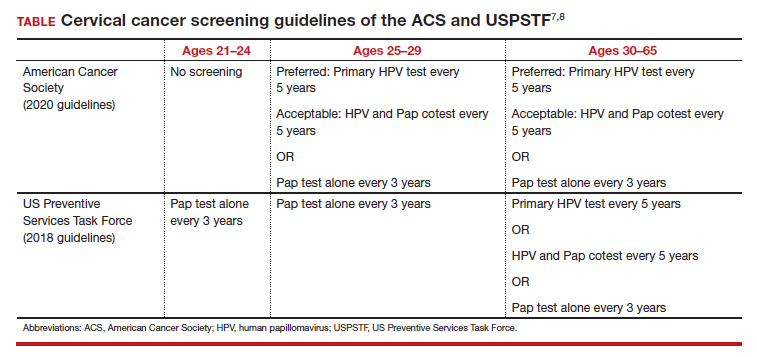
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22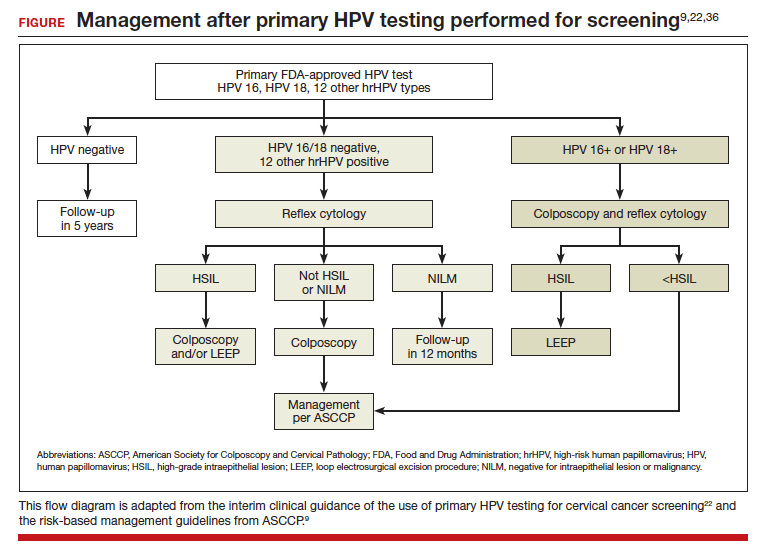
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
2022 Update on cervical disease
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12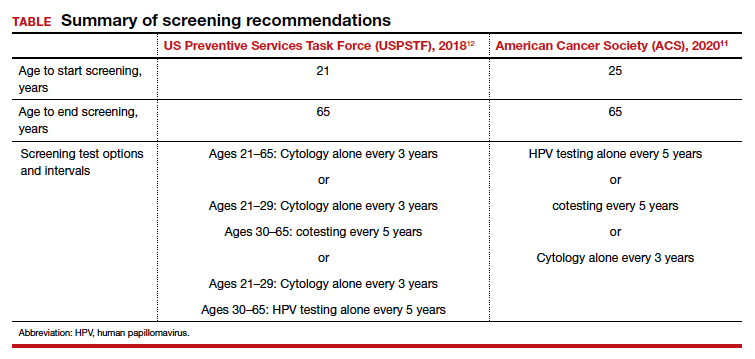
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.
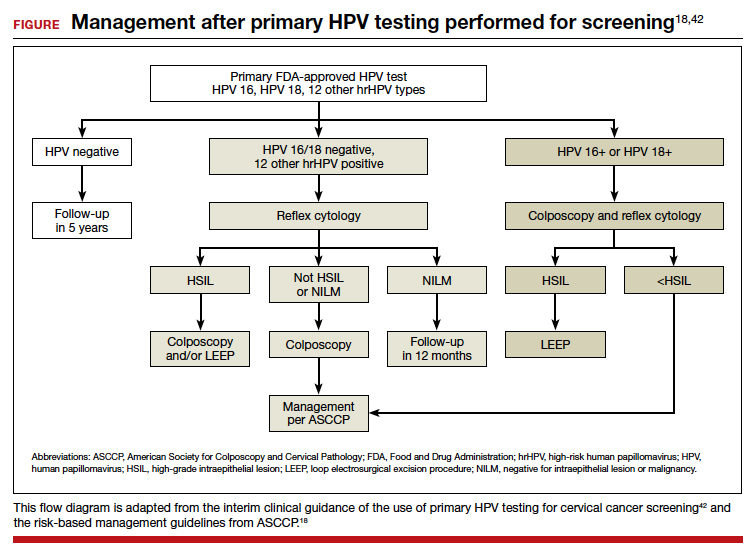
HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
2021 Update on cervical disease
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.
Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.
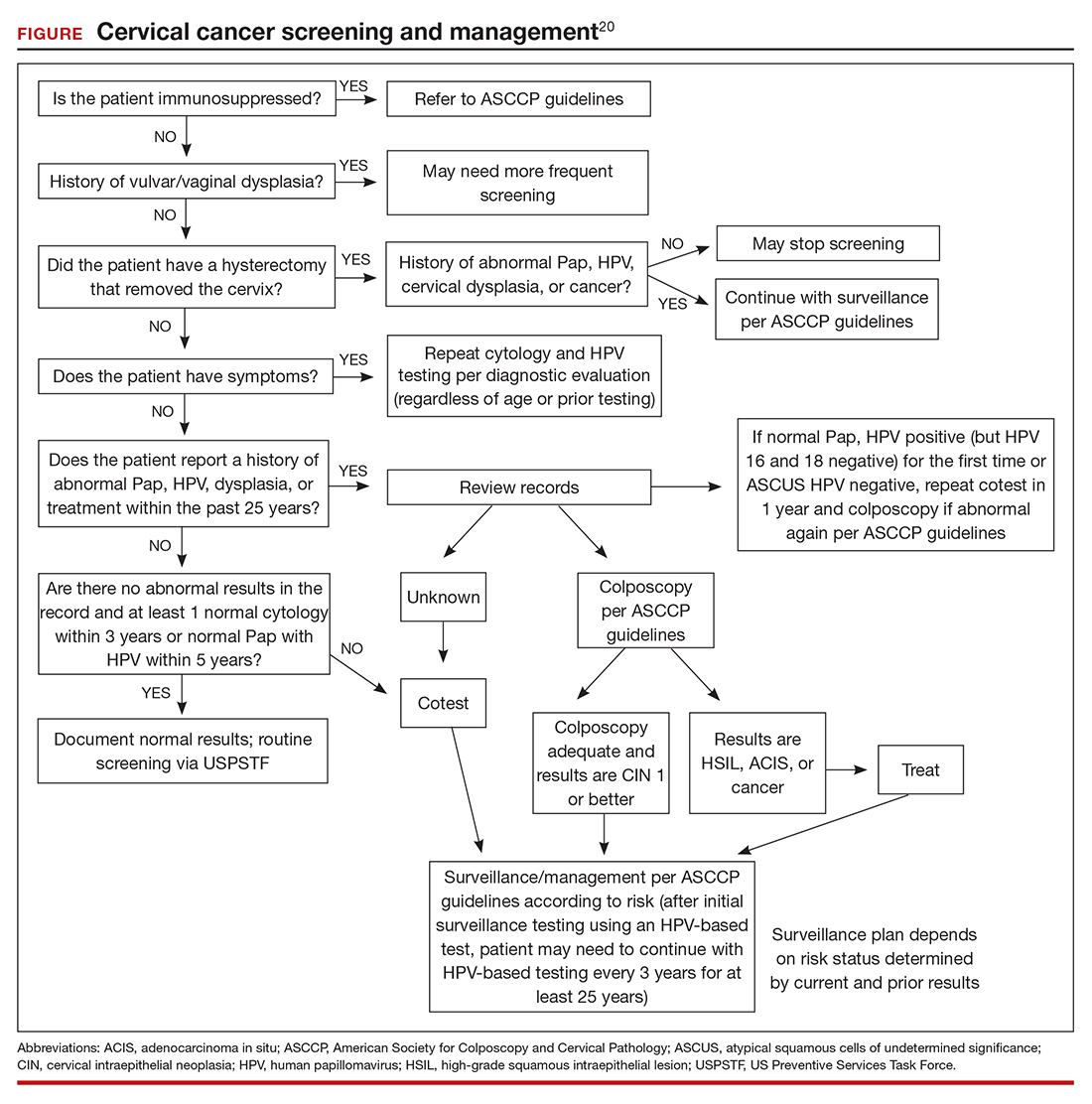
Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Are we ready for primary HPV testing for the prevention of cervical cancer?
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.