User login
Persistent Chlorotrichosis With Chronic Sun Exposure
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
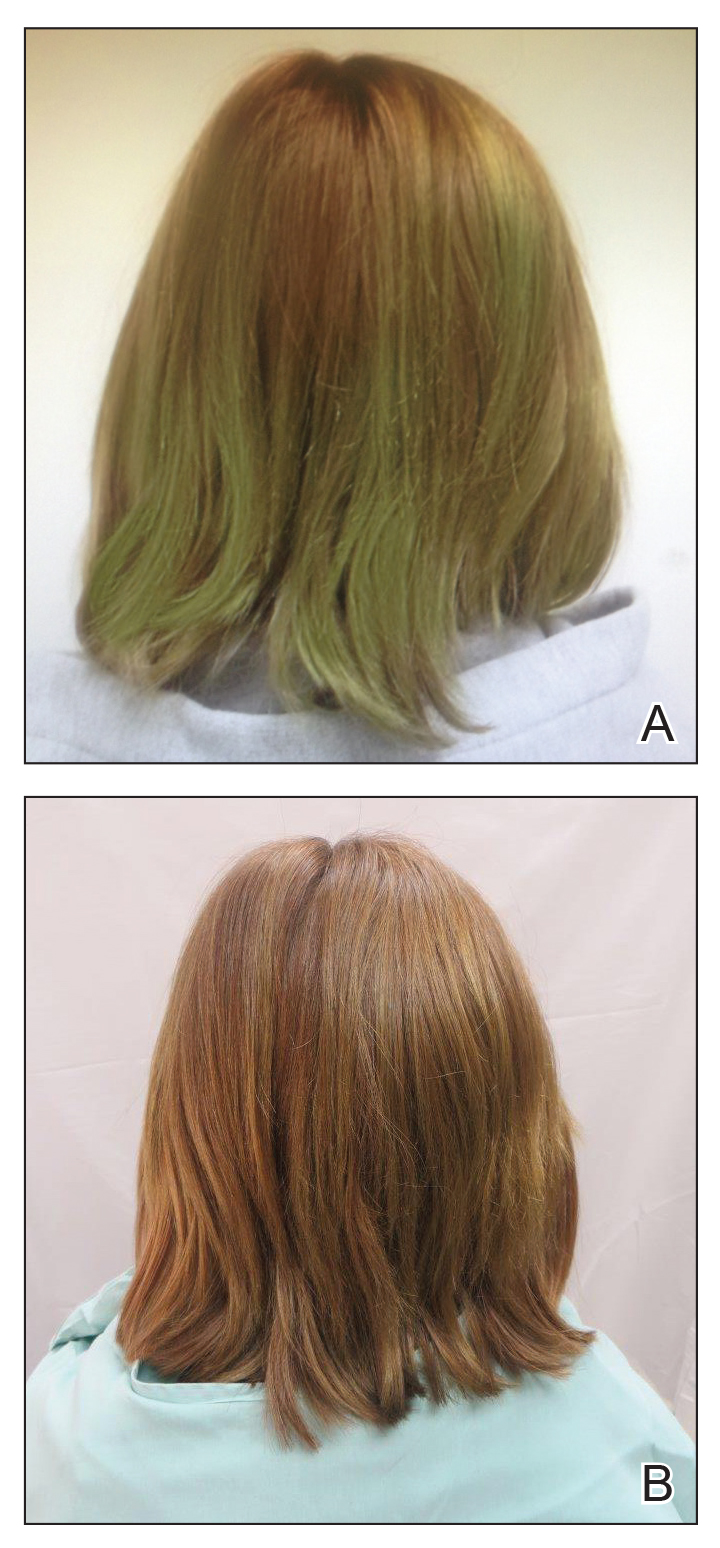
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
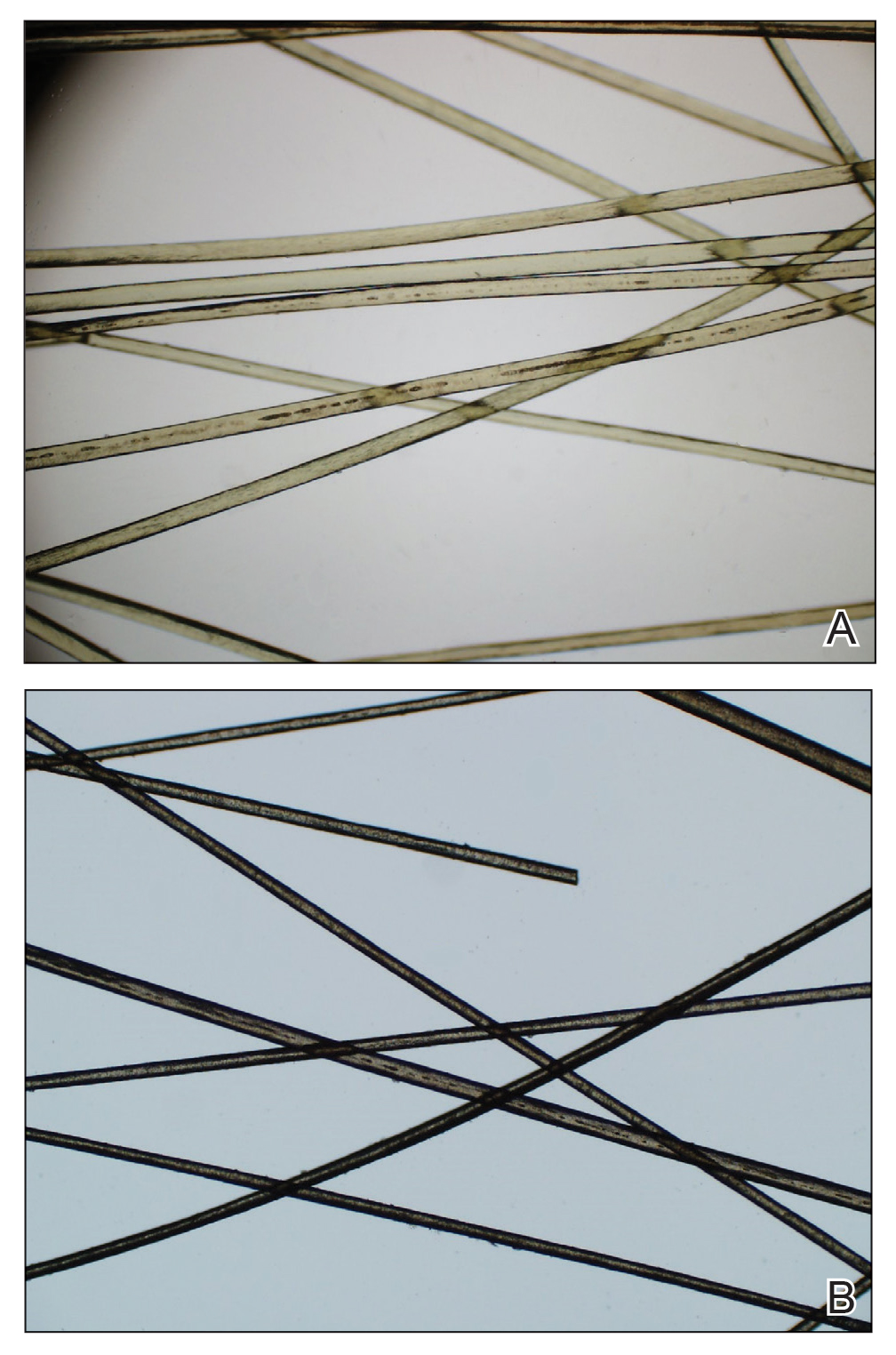
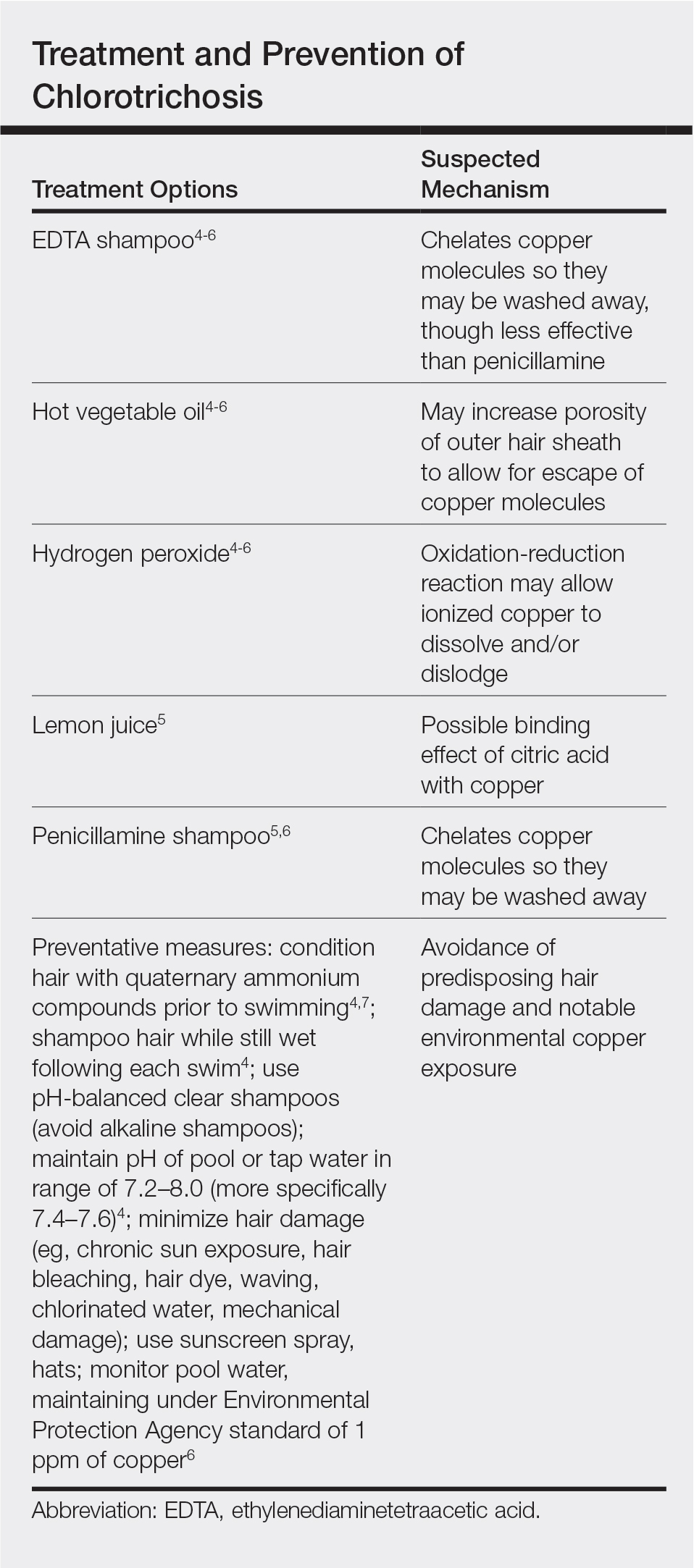
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
Practice Points
- Chlorotrichosis is the deposition of copper onto hair, which causes a green discoloration and most commonly occurs in blonde patients with excessive exposure to chlorinated water.
- Hair cuticle damage from hair care practices, such as use of heat or chemicals, can predispose patients to the development of chlorotrichosis.
- Although a number of treatments have been proposed, the use of penicillamine shampoo seems to be particularly effective and works via chelation of the adherent copper molecules.
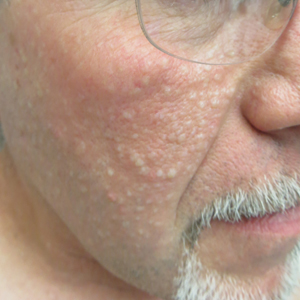
Multiple Facial Papules
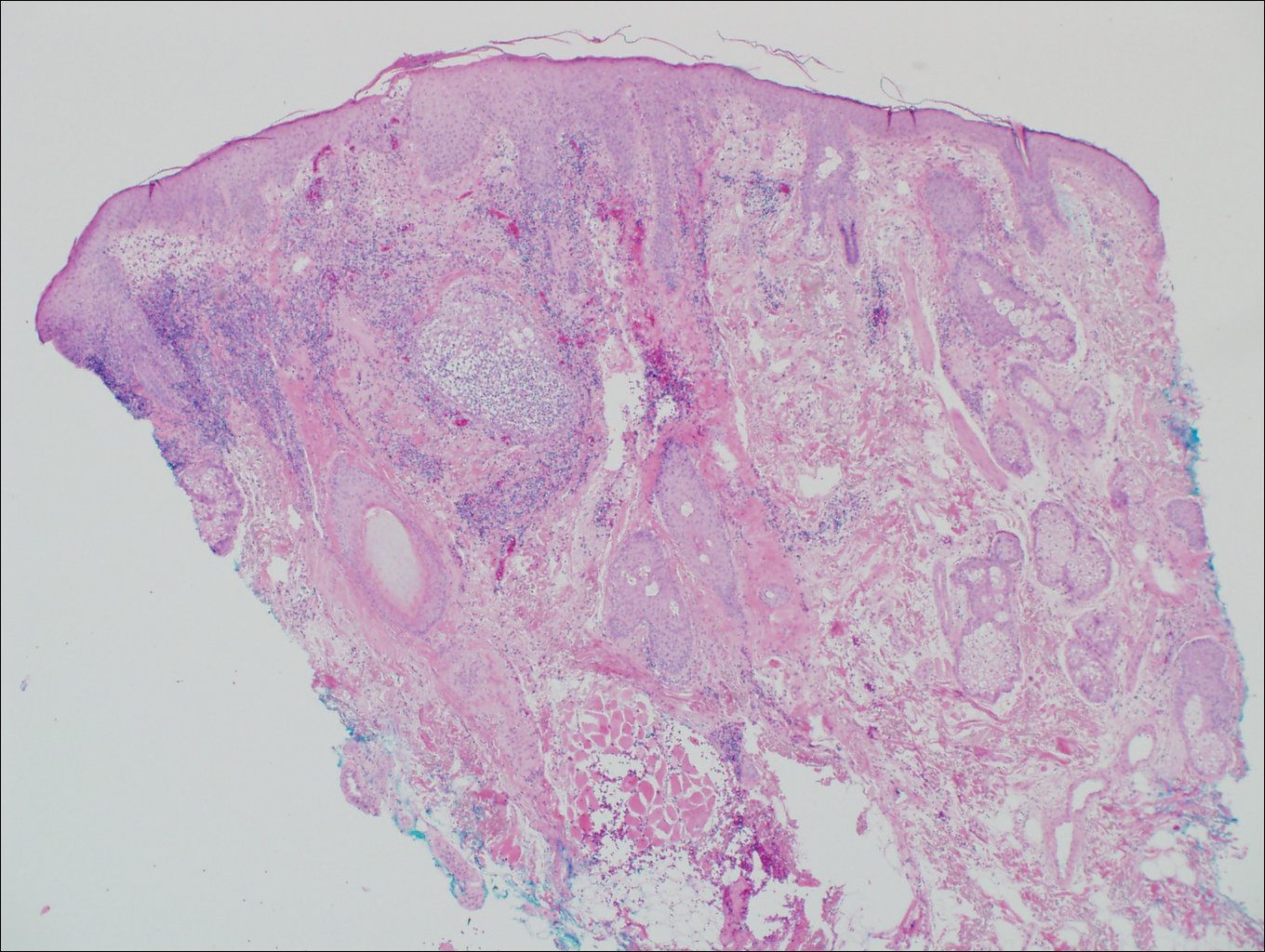
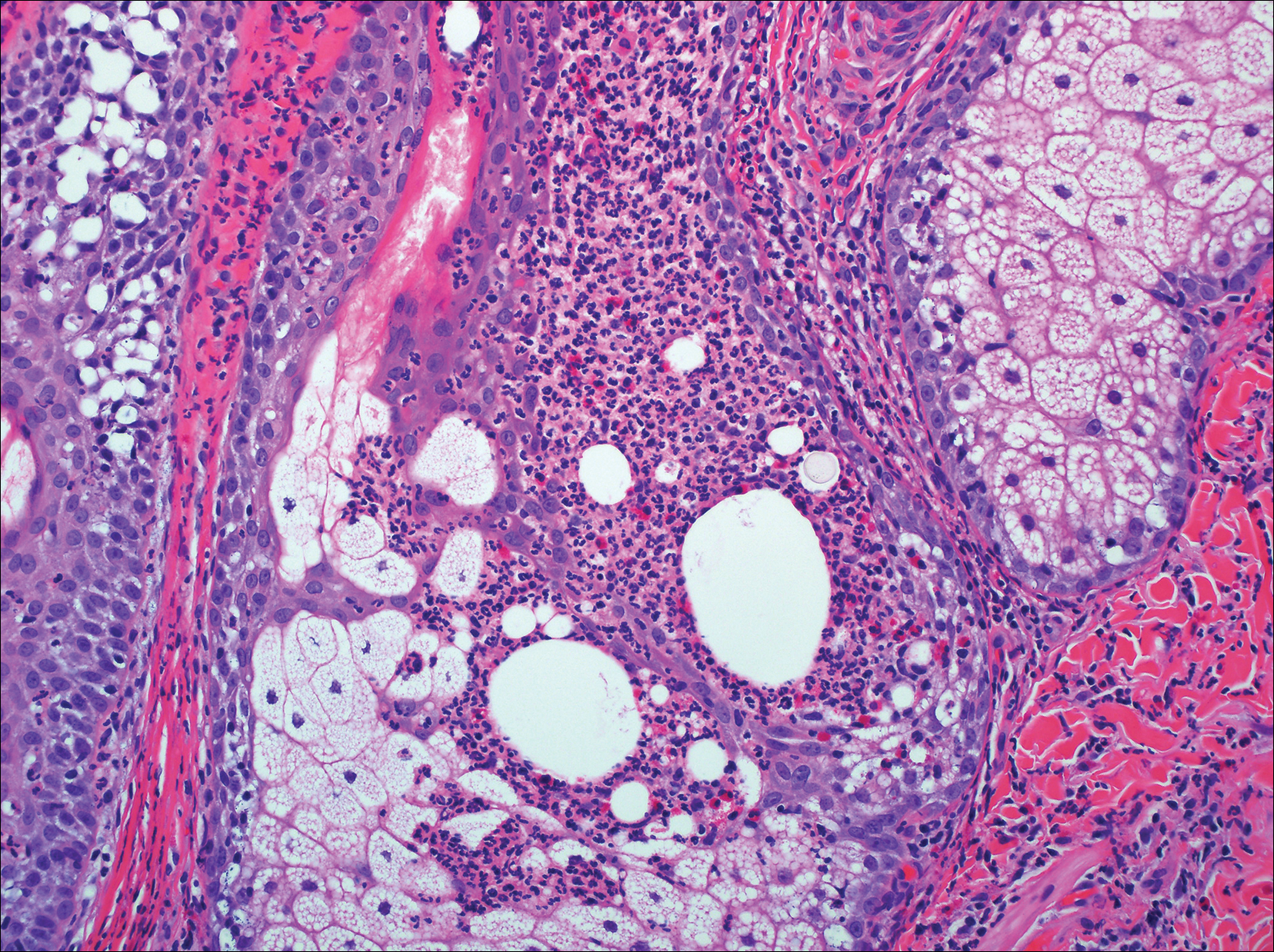
The Diagnosis: Birt-Hogg-Dubé Syndrome

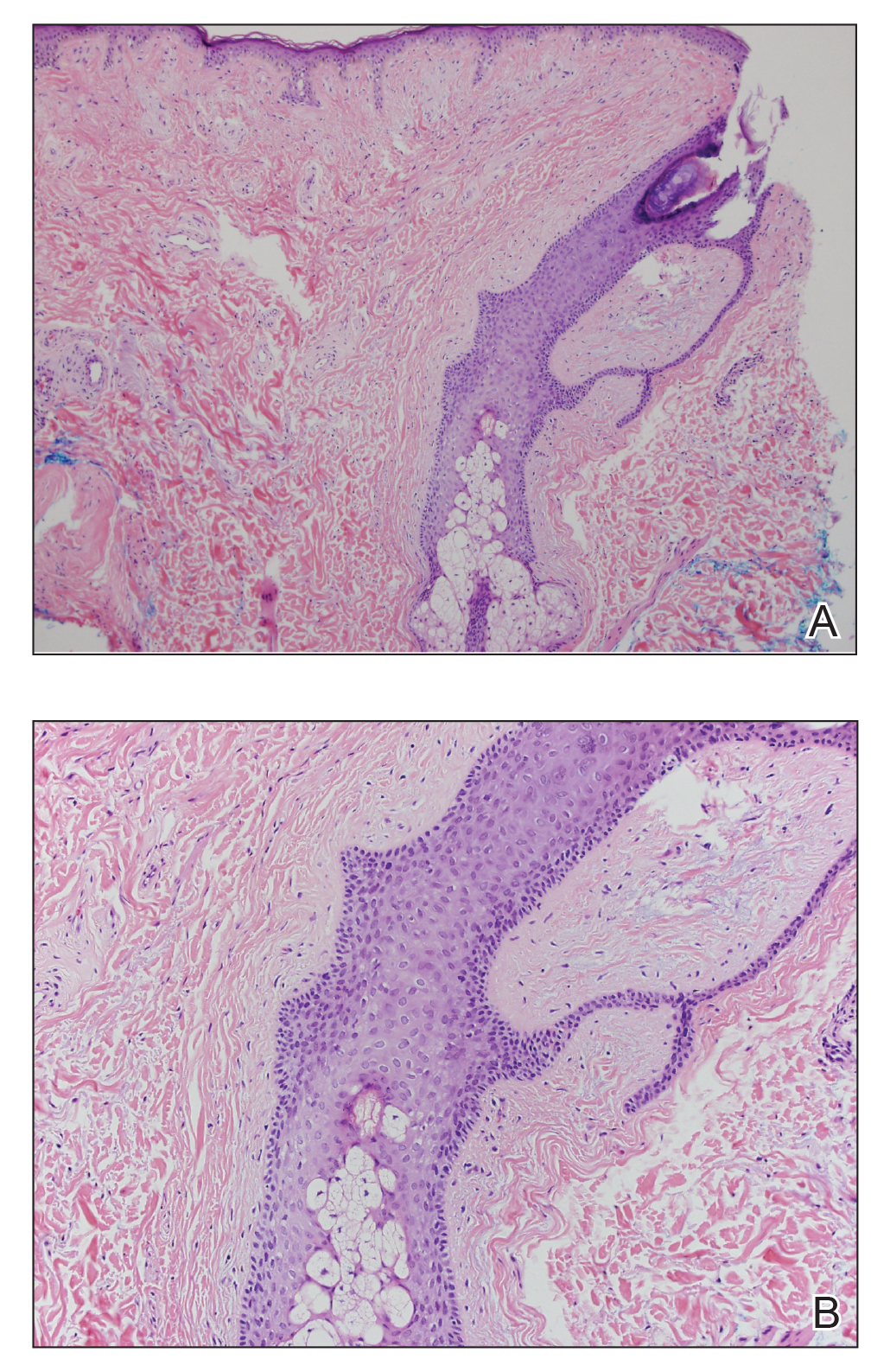
Histopathologic examination revealed a collection of bland spindle cells with perifollicular fibrosis consistent with a fibrofolliculoma, confirming the diagnosis of Birt-Hogg-Dubé syndrome (Figure). Cosmetic treatment with ablative therapy was offered, but the patient declined.
Birt-Hogg-Dubé syndrome is an autosomal-dominant genodermatosis caused by a loss-of-function mutation in the folliculin gene, FLCN, on chromosome arm 17p11.2.1 Cutaneous findings include benign follicular hamartomas, such as fibrofolliculomas and trichodiscomas. Angiofibromas, perifollicular fibromas, oral papillomas, and acrochordons also can be present.1 Cutaneous lesions usually appear on the head and neck in the third decade of life.
Patients with Birt-Hogg-Dubé syndrome are at an increased risk for pneumothorax and renal cancer, specifically hybrid oncocytic-chromophobe renal cell carcinomas.2 In a study of 89 patients with a FLCN mutation, 90% (80/89) of patients had cutaneous lesions, 84% (34/89) had pulmonary cysts, and 34% (30/89) had kidney tumors. Affected individuals were at a higher risk for pneumothorax and kidney tumors if there was a family history of these tumors.2
Proposed diagnostic criteria include any 1 of the following: 2 or more skin lesions clinically consistent with fibrofolliculomas and 1 histologically confirmed fibrofolliculoma; multiple bilateral pulmonary cysts in the basilar lung with or without pneumothorax before 40 years of age; bilateral multifocal chromophobe renal carcinomas or hybrid oncocytic tumors; combination of cutaneous, pulmonary, or renal manifestation in the patient and family; or a FLCN mutation.3
Current recommendations for the workup of a patient with Birt-Hogg-Dubé syndrome include referral to genetic counseling for the patient and family, a baseline computed tomography of the chest to evaluate for pulmonary cysts, and gadolinium-enhanced abdominal magnetic resonance imaging starting at 20 years of age and repeated every 3 to 4 years to screen for renal tumors.1 Pulmonary function tests can be considered if the patient is symptomatic or has a high cyst burden. Patients should be advised against smoking and scuba diving.
The differential diagnosis of multiple facial papules includes Cowden syndrome, tuberous sclerosis, Brooke-Spiegler syndrome, and Muir-Torre syndrome. Cowden syndrome is caused by a mutation in the protein tyrosine phosphatase gene, PTEN.4 The characteristic cutaneous findings on the face are trichilemmomas, which appear as flesh-colored papules that may have a verrucous surface.
Tuberous sclerosis is caused by mutations in hamartin (TSC1) or tuberin (TSC2). Angiofibromas are most commonly found on the face and appear as flesh-colored to red-brown papules. Fibrous plaques, periungual fibromas, gingival fibromas, hypopigmented macules, and connective tissue nevi also are found in tuberous sclerosis.5
Brooke-Spiegler syndrome is caused by a mutation in the CYLD lysine 63 deubiquitinase gene, CYLD. Trichoepitheliomas, cylindromas, and spiradenomas are caused by the CYLD mutation and appear on the head and neck. Trichoepitheliomas are flesh-colored to pink papules found on the face, often concentrated in the nasolabial folds.6 Cylindromas and spiradenomas are flesh-colored to pink papules or nodules most commonly found on the scalp.6
Muir-Torre syndrome is caused by a mutation in DNA mismatch repair genes MSH2 and/or MLH1.7 Sebaceous neoplasms, including sebaceous adenomas, sebaceomas, and less frequently sebaceous carcinomas, are characteristic cutaneous findings and appear as pink to yellow papules commonly found on the head and neck.
Careful history taking, physical examination, and histopathologic analysis are important in recognizing the features of Birt-Hogg-Dubé syndrome. Accurate and timely diagnosis is essential for the appropriate care of patients and their families, given the syndrome's systemic implications.
- Gupta N, Sunwoo BY, Kotloff RM. Birt-Hogg-Dubé syndrome. Clin Chest Med. 2016;37:475-486.
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321-331.
- Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558-569.
- Marsh D, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461-1472.
- Wataya-Kaneda M, Uemura M, Fujita K, et al. Tuberous sclerosis complex: recent advances in manifestations and therapy. Int J Urol. 2017;24:681-691.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Mahalingam M. MSH6, Past and present and Muir-Torre syndrome--connecting the dots. Am J Dermatopathol. 2017;39:239-249.

The Diagnosis: Birt-Hogg-Dubé Syndrome

Histopathologic examination revealed a collection of bland spindle cells with perifollicular fibrosis consistent with a fibrofolliculoma, confirming the diagnosis of Birt-Hogg-Dubé syndrome (Figure). Cosmetic treatment with ablative therapy was offered, but the patient declined.
Birt-Hogg-Dubé syndrome is an autosomal-dominant genodermatosis caused by a loss-of-function mutation in the folliculin gene, FLCN, on chromosome arm 17p11.2.1 Cutaneous findings include benign follicular hamartomas, such as fibrofolliculomas and trichodiscomas. Angiofibromas, perifollicular fibromas, oral papillomas, and acrochordons also can be present.1 Cutaneous lesions usually appear on the head and neck in the third decade of life.
Patients with Birt-Hogg-Dubé syndrome are at an increased risk for pneumothorax and renal cancer, specifically hybrid oncocytic-chromophobe renal cell carcinomas.2 In a study of 89 patients with a FLCN mutation, 90% (80/89) of patients had cutaneous lesions, 84% (34/89) had pulmonary cysts, and 34% (30/89) had kidney tumors. Affected individuals were at a higher risk for pneumothorax and kidney tumors if there was a family history of these tumors.2
Proposed diagnostic criteria include any 1 of the following: 2 or more skin lesions clinically consistent with fibrofolliculomas and 1 histologically confirmed fibrofolliculoma; multiple bilateral pulmonary cysts in the basilar lung with or without pneumothorax before 40 years of age; bilateral multifocal chromophobe renal carcinomas or hybrid oncocytic tumors; combination of cutaneous, pulmonary, or renal manifestation in the patient and family; or a FLCN mutation.3
Current recommendations for the workup of a patient with Birt-Hogg-Dubé syndrome include referral to genetic counseling for the patient and family, a baseline computed tomography of the chest to evaluate for pulmonary cysts, and gadolinium-enhanced abdominal magnetic resonance imaging starting at 20 years of age and repeated every 3 to 4 years to screen for renal tumors.1 Pulmonary function tests can be considered if the patient is symptomatic or has a high cyst burden. Patients should be advised against smoking and scuba diving.
The differential diagnosis of multiple facial papules includes Cowden syndrome, tuberous sclerosis, Brooke-Spiegler syndrome, and Muir-Torre syndrome. Cowden syndrome is caused by a mutation in the protein tyrosine phosphatase gene, PTEN.4 The characteristic cutaneous findings on the face are trichilemmomas, which appear as flesh-colored papules that may have a verrucous surface.
Tuberous sclerosis is caused by mutations in hamartin (TSC1) or tuberin (TSC2). Angiofibromas are most commonly found on the face and appear as flesh-colored to red-brown papules. Fibrous plaques, periungual fibromas, gingival fibromas, hypopigmented macules, and connective tissue nevi also are found in tuberous sclerosis.5
Brooke-Spiegler syndrome is caused by a mutation in the CYLD lysine 63 deubiquitinase gene, CYLD. Trichoepitheliomas, cylindromas, and spiradenomas are caused by the CYLD mutation and appear on the head and neck. Trichoepitheliomas are flesh-colored to pink papules found on the face, often concentrated in the nasolabial folds.6 Cylindromas and spiradenomas are flesh-colored to pink papules or nodules most commonly found on the scalp.6
Muir-Torre syndrome is caused by a mutation in DNA mismatch repair genes MSH2 and/or MLH1.7 Sebaceous neoplasms, including sebaceous adenomas, sebaceomas, and less frequently sebaceous carcinomas, are characteristic cutaneous findings and appear as pink to yellow papules commonly found on the head and neck.
Careful history taking, physical examination, and histopathologic analysis are important in recognizing the features of Birt-Hogg-Dubé syndrome. Accurate and timely diagnosis is essential for the appropriate care of patients and their families, given the syndrome's systemic implications.

The Diagnosis: Birt-Hogg-Dubé Syndrome

Histopathologic examination revealed a collection of bland spindle cells with perifollicular fibrosis consistent with a fibrofolliculoma, confirming the diagnosis of Birt-Hogg-Dubé syndrome (Figure). Cosmetic treatment with ablative therapy was offered, but the patient declined.
Birt-Hogg-Dubé syndrome is an autosomal-dominant genodermatosis caused by a loss-of-function mutation in the folliculin gene, FLCN, on chromosome arm 17p11.2.1 Cutaneous findings include benign follicular hamartomas, such as fibrofolliculomas and trichodiscomas. Angiofibromas, perifollicular fibromas, oral papillomas, and acrochordons also can be present.1 Cutaneous lesions usually appear on the head and neck in the third decade of life.
Patients with Birt-Hogg-Dubé syndrome are at an increased risk for pneumothorax and renal cancer, specifically hybrid oncocytic-chromophobe renal cell carcinomas.2 In a study of 89 patients with a FLCN mutation, 90% (80/89) of patients had cutaneous lesions, 84% (34/89) had pulmonary cysts, and 34% (30/89) had kidney tumors. Affected individuals were at a higher risk for pneumothorax and kidney tumors if there was a family history of these tumors.2
Proposed diagnostic criteria include any 1 of the following: 2 or more skin lesions clinically consistent with fibrofolliculomas and 1 histologically confirmed fibrofolliculoma; multiple bilateral pulmonary cysts in the basilar lung with or without pneumothorax before 40 years of age; bilateral multifocal chromophobe renal carcinomas or hybrid oncocytic tumors; combination of cutaneous, pulmonary, or renal manifestation in the patient and family; or a FLCN mutation.3
Current recommendations for the workup of a patient with Birt-Hogg-Dubé syndrome include referral to genetic counseling for the patient and family, a baseline computed tomography of the chest to evaluate for pulmonary cysts, and gadolinium-enhanced abdominal magnetic resonance imaging starting at 20 years of age and repeated every 3 to 4 years to screen for renal tumors.1 Pulmonary function tests can be considered if the patient is symptomatic or has a high cyst burden. Patients should be advised against smoking and scuba diving.
The differential diagnosis of multiple facial papules includes Cowden syndrome, tuberous sclerosis, Brooke-Spiegler syndrome, and Muir-Torre syndrome. Cowden syndrome is caused by a mutation in the protein tyrosine phosphatase gene, PTEN.4 The characteristic cutaneous findings on the face are trichilemmomas, which appear as flesh-colored papules that may have a verrucous surface.
Tuberous sclerosis is caused by mutations in hamartin (TSC1) or tuberin (TSC2). Angiofibromas are most commonly found on the face and appear as flesh-colored to red-brown papules. Fibrous plaques, periungual fibromas, gingival fibromas, hypopigmented macules, and connective tissue nevi also are found in tuberous sclerosis.5
Brooke-Spiegler syndrome is caused by a mutation in the CYLD lysine 63 deubiquitinase gene, CYLD. Trichoepitheliomas, cylindromas, and spiradenomas are caused by the CYLD mutation and appear on the head and neck. Trichoepitheliomas are flesh-colored to pink papules found on the face, often concentrated in the nasolabial folds.6 Cylindromas and spiradenomas are flesh-colored to pink papules or nodules most commonly found on the scalp.6
Muir-Torre syndrome is caused by a mutation in DNA mismatch repair genes MSH2 and/or MLH1.7 Sebaceous neoplasms, including sebaceous adenomas, sebaceomas, and less frequently sebaceous carcinomas, are characteristic cutaneous findings and appear as pink to yellow papules commonly found on the head and neck.
Careful history taking, physical examination, and histopathologic analysis are important in recognizing the features of Birt-Hogg-Dubé syndrome. Accurate and timely diagnosis is essential for the appropriate care of patients and their families, given the syndrome's systemic implications.
- Gupta N, Sunwoo BY, Kotloff RM. Birt-Hogg-Dubé syndrome. Clin Chest Med. 2016;37:475-486.
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321-331.
- Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558-569.
- Marsh D, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461-1472.
- Wataya-Kaneda M, Uemura M, Fujita K, et al. Tuberous sclerosis complex: recent advances in manifestations and therapy. Int J Urol. 2017;24:681-691.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Mahalingam M. MSH6, Past and present and Muir-Torre syndrome--connecting the dots. Am J Dermatopathol. 2017;39:239-249.
- Gupta N, Sunwoo BY, Kotloff RM. Birt-Hogg-Dubé syndrome. Clin Chest Med. 2016;37:475-486.
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321-331.
- Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558-569.
- Marsh D, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461-1472.
- Wataya-Kaneda M, Uemura M, Fujita K, et al. Tuberous sclerosis complex: recent advances in manifestations and therapy. Int J Urol. 2017;24:681-691.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Mahalingam M. MSH6, Past and present and Muir-Torre syndrome--connecting the dots. Am J Dermatopathol. 2017;39:239-249.
A 50-year-old man presented with facial papules on the cheeks that had appeared approximately 1.5 years prior and gradually spread over the face and neck. They were occasionally pruritic but otherwise were asymptomatic. His mother and brother reportedly had similar clinical findings. Family history was notable for a maternal uncle who had died in his 30s of an unknown type of renal cancer. Physical examination revealed innumerable white-gray papules that measured 1 to 5 mm and were scattered across the face and neck. Punch biopsies were obtained. Computed tomography of the chest showed multiple bibasilar pulmonary cysts. Magnetic resonance imaging was negative for renal tumors.
Pruritic Papules on the Scalp and Arms
Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1
Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
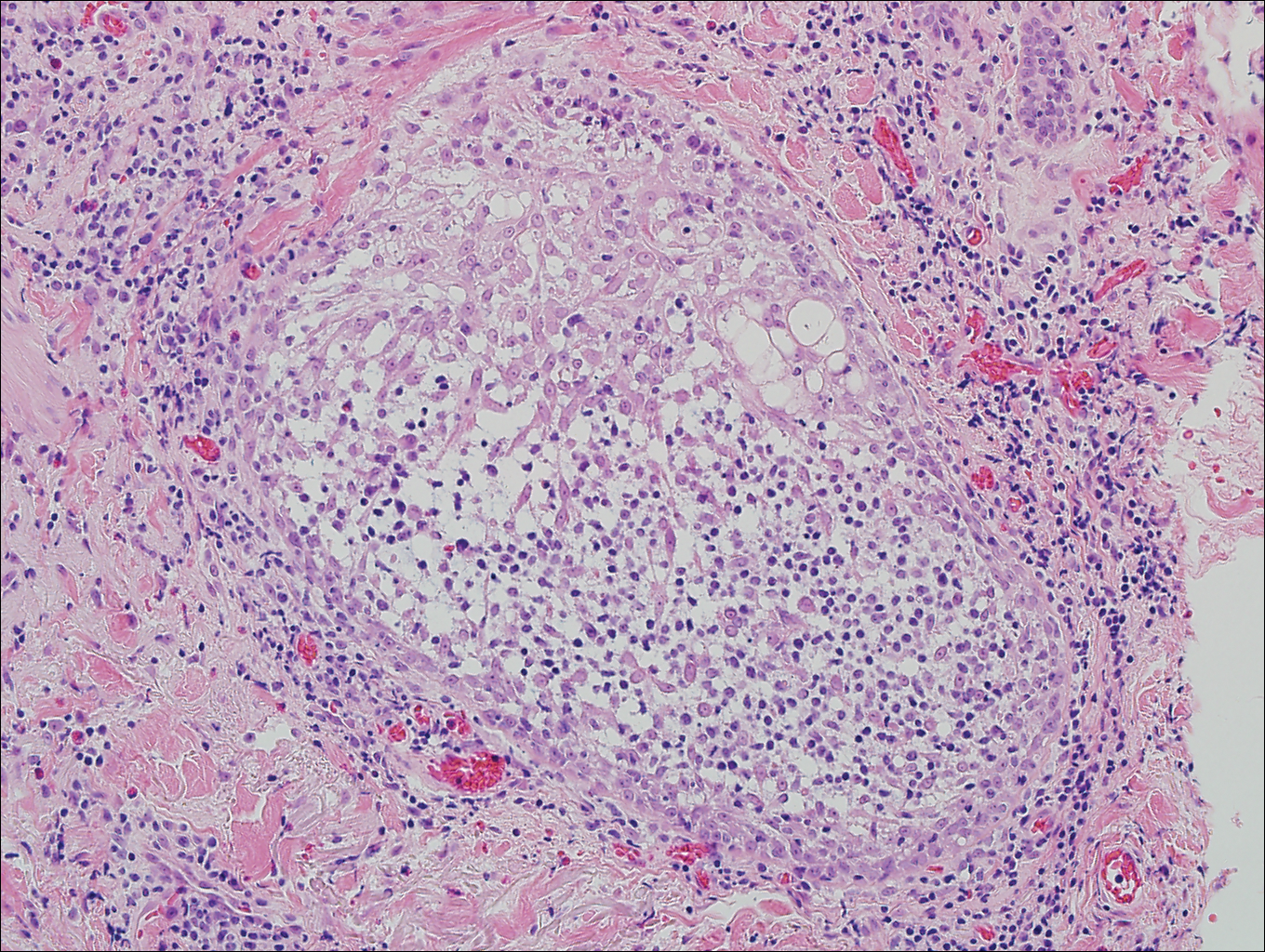
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.
Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7
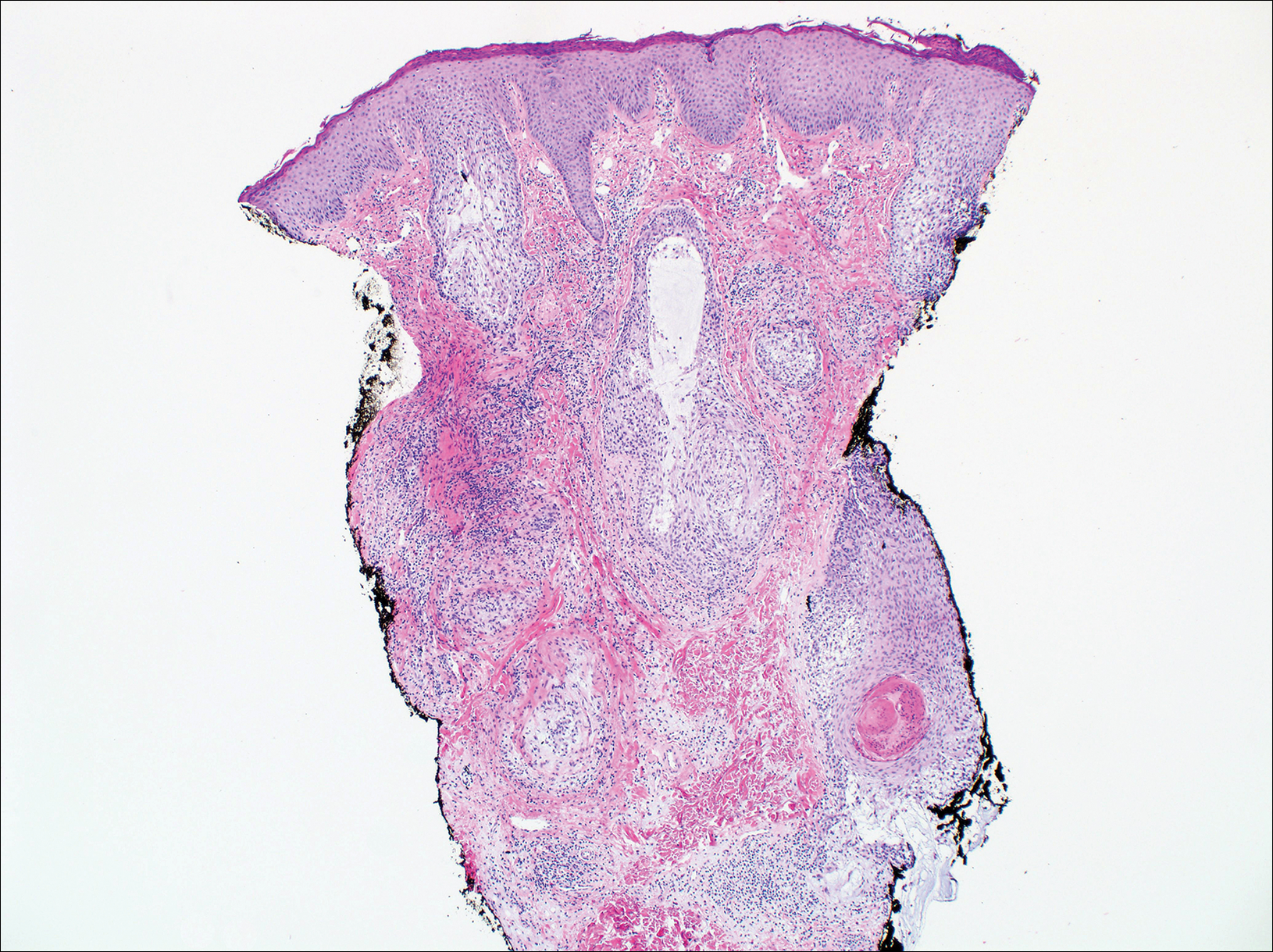
Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.
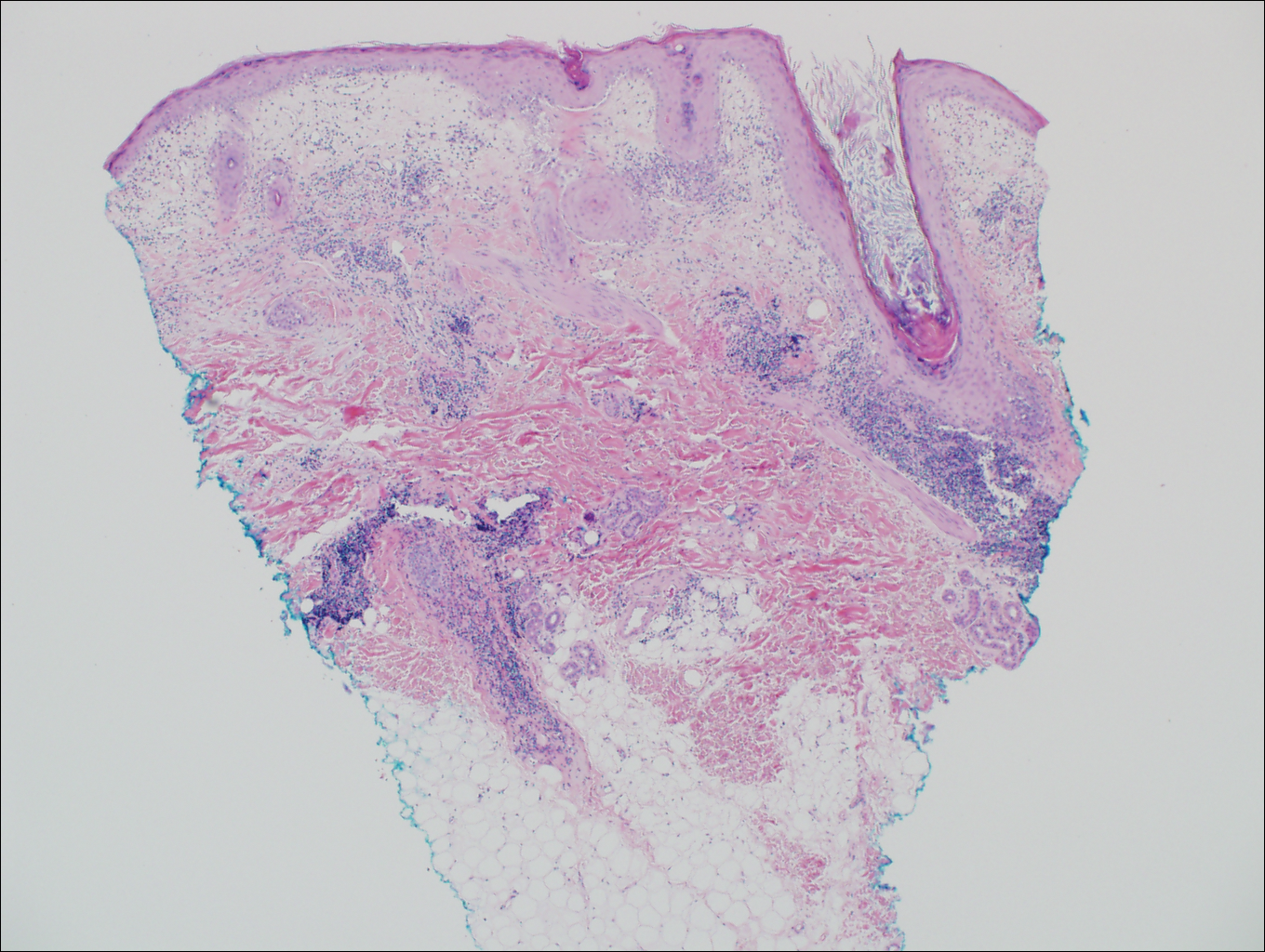
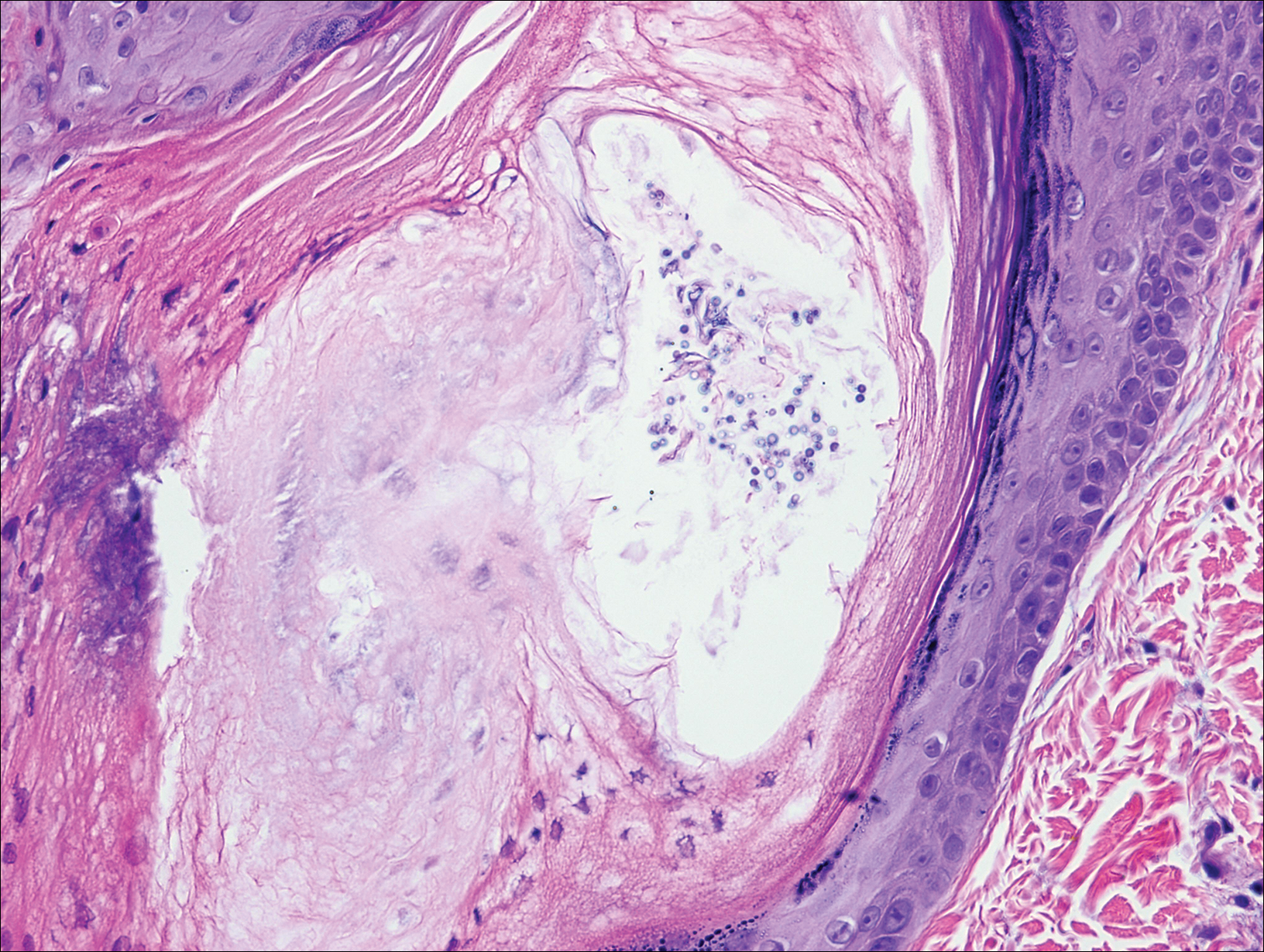
Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1

Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.

Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7

Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.

Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10

Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1

Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.

Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7

Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.

Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10

- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
A 60-year-old man presented with a 3-month history of itchy bumps on the scalp and arms. He also noticed some patches of hair loss in these areas. He had no history of other skin conditions and was otherwise healthy with no other medical comorbidities.