User login
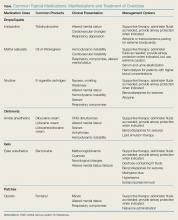
Drops, Ointments, Gels, and Patches: The Dangers of Topical Medications
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Te
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
1. Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol. 2016;54(10):924-1109. doi:10.1080/15563650.2016.1245421.
2. Tobias JD. Central nervous system depression following accidental ingestion of visine eye drops. Clin Pediatr (Phila). 1996;35(10):539-540. doi:10.1177/000992289603501010.
3. Lev R, Clark RF. Visine overdose: case report of an adult with hemodynamic compromise. J Emerg Med. 1995;13(5):649-652.
4. Jensen P, Edgren B, Hall L, Ring JC. Hemodynamic effects following ingestion of an imidazoline-containing product. Pediatr Emerg Care. 1989;5(2):110-112.
5. Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32(1):63-69. doi:10.1016/j.jemermed.2006.08.009.
6. Chan TY. The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. Postgrad Med J. 1996;72(844):109-112.
7. Juurlink DN, Gosselin S, Kielstein JT, et al. Extracorporeal treatment for salicylate poisoning: Systematic review and recommendations from the EXTRIP workgroup. Ann Emerg Med. 2015;66(2):165-181.
8. Manikian A, Stone S, Hamilton R, Foltin G, Howland MA, Hoffman RS. Exchange transfusion in severe infant salicylism. Vet Hum Toxicol. 2002;44(4):224-227.
9. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134-141. doi:10.1093/ntr/ntu080.
10. US Food & Drug Administration. Tobacco Products. Rules & Regulations. https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm283974.htm. Updated February 16, 2017. Accessed March 7, 2017.
11. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5-7. doi:10.1007/s00204-013-1127-0.
12. Bassett RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370(23):2249-2250. doi:10.1056/NEJMc1403843.
13. Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517-521; quiz 522-524. doi:10.1097/PEC.0000000000000486.
14. Wain AA, Martin J. Can transdermal nicotine patch cause acute intoxication in a child? A case report and review of literature. Ulster Med J. 2004;73(1):65-66.
15. Gill N, Sangha G, Poonai N, Lim R. E-Cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699-703. doi:10.1017/cem.2015.10.
16. Dayan PS, Litovitz TL, Crouch BI, Scalzo AJ, Klein BL. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28(4):442-445.
17. Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37(1):32-39. doi:10.1016/j.jemermed.2007.11.005.
18. Ciechanowicz S, Patil V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol Res Pract. 2012;2012:131784. doi:10.1155/2012/131784.
19. Bong CL, Hilliard J, Seefelder C. Severe methemoglobinemia from topical benzocaine 7.5% (baby orajel) use for teething pain in a toddler. Clin Pediatr (Phila). 2009;48(2):209-211.
20. Chung N, Batra R, Itzkevitch M, Boruchov D, Baldauf M. Severe methemoglobinemia linked to gel-type topical benzocaine use: A case report. J Emerg Med. 2010;38(5):601-606. doi:10.1016/j.jemermed.2008.06.025.
21. Liebelt EL, Shannon MW. Small doses, big problems: A selected review of highly toxic common medications. Pediatr Emerg Care. 1993;9(5):292-297.
22. So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335-339; quiz 340-341. doi:10.1016/j.pedhc.2008.08.008.
23. Parekh D, Miller MA, Borys D, Patel PR, Levsky ME. Transdermal patch medication delivery systems and pediatric poisonings, 2002-2006. Clin Pediatr (Phila). 2008;47(7):659-663. doi:10.1177/0009922808315211.
24. Teske J, Weller JP, Larsch K, Tröger HD, Karst M. Fatal outcome in a child after ingestion of a transdermal fentanyl patch. Int J Legal Med. 2007;121(2):147-151. doi:10.1007/s00414-006-0137-3.
25. Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969-971. doi:10.1177/106002809502901001.
26. Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938. doi:10.1155/2011/495938.
27. Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6(4):443-447. doi:10.1007/s13181-010-0092-8.
28. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
29 Mrvos R, Feuchter AC, Katz KD, Duback-Morris LF, Brooks DE, Krenzelok EP. Whole fentanyl patch ingestion: A multi-center case series. J Emerg Med. 2012;42(5):549-552. doi:10.1016/j.jemermed.2011.05.017.
30. Sachdeva DK, Stadnyk JM. Are one or two dangerous? Opioid exposure in toddlers. J Emerg Med. 2005;29(1):77-84. doi:10.1016/j.jemermed.2004.12.015.
31. Behrman A, Goertemoeller S. A sticky situation: toxicity of clonidine and fentanyl transdermal patches in pediatrics. J Emerg Nurs. 2007;33(3):290-293.doi: 10.1016/j.jen.2007.02.004.
He Huffed and He Puffed and He Got Frostbite
Case
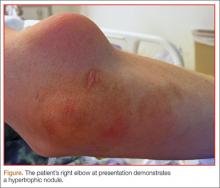
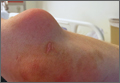
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
Case
A 27-year-old man presented to an ED after experiencing a syncopal episode. His vital signs at presentation were normal. Physical examination was generally normal except that there were blisters on the patient’s abdomen, left hand, and right arm, as well as a hypertrophic nodule on the right elbow (Figure) and hard growths on the digits of the right hand. The patient stated the growths started 5 months ago and had been increasing in size. On further questioning, the patient admitted to “huffing” (ie, inhaling) at least six cans of pressurized dust-removal keyboard cleaning spray daily for the past 11 months.
Why do patients abuse keyboard cleaning spray?
The propellant used in certain liquefied compressed gas products is 1,1-difluoroethane (1,1-DFE), a fluorinated hydrocarbon. It is a member of a broad class of related compounds that are present in spray paints, glues, nail polish removers, fuels, hair sprays, and air-freshening products. These 1,1-DFE-containing products are abused for their rapid and short-acting central nervous system (CNS) depressant effects—not unlike that of ethanol. Typically, the vapor of a volatile hydrocarbon is inhaled directly from the open container (“sniffing”), from a bag (“bagging”), or from a soaked rag (huffing). Not only are such hydrocarbon-containing products easy to conceal, they are also highly accessible and inexpensive. Moreover, there are generally no direct legal consequences resulting from abuse of these substances.
All of the aforementioned factors make hydrocarbons a popular drug of abuse among adolescents. Approximately 75% of the population abusing hydrocarbons is younger than age 18 years, half of whom reported first use prior to age 13 years.1,2 Though inhalant abuse rarely continues into adulthood, 0.1% of individuals between the ages of 18 and 30 years report having an inhalant-use disorder.
Hydrocarbons and their halogenated derivatives are lipophilic compounds that are rapidly absorbed after inhalation and rapidly distributed to CNS and cardiac tissue. The brain concentration of 1,1-DFE likely peaks higher than concentrations in other organs and is cleared more rapidly.3 Hydrocarbons produce CNS depression secondary to multiple mechanisms, including gamma-aminobutyric acid agonism, dopamine modulation, and N-methyl-D-aspartate-receptor antagonism.4,5
What causes skin lesions on the abdomen and arms?
The lesions on the patient’s abdomen and extremities were consistent with frostbite. The liquefied compressed gas in computer-cleaning and related products is housed in a pressurized canister. The pressure is released when the spray nozzle is depressed; this causes the liquid to rapidly expand to a gas as it is released, resulting in a quick decrease in the temperature of the metal canister. This process, referred to as adiabatic cooling, demonstrates the first law of thermodynamics. The cold temperature of both the liquid and the canister can cause frostbite in the digits and other parts of the body with which the canister or liquid comes into contact.6
Why did the patient have syncope?
Halogenated hydrocarbons inhibit the cardiac delayed rectifier potassium channels involved in the repolarization of cardiac myocytes, causing a delay in repolarization that is manifested as prolongation of the QT interval on an electrocardiogram. This condition places patients at an increased risk of developing torsades de pointes (TdP).7 In most cases, TdP is self-terminating; however, if TdP persists, degeneration to ventricular fibrillation will result. Deaths caused in this fashion have been referred to as “sudden sniffing death syndrome,” and account for half of all hydrocarbon-related deaths.6,8 In addition to the cardiac effects, hydrocarbons are simple asphyxiants that act by displacing oxygen from inspired air, which also contributes to syncope.
It is important to note that epinephrine and other catecholamines increase the risk for dysrhythmias such as TdP in the setting of hydrocarbon abuse.9 For this reason, epinephrine should be used with caution in the setting of a hydrocarbon-induced arrhythmia. Beta-adrenergic antagonists such as esmolol and propranolol are preferable because they reduce the incidence of ectopia that may trigger TdP.10
What is the significance of the masses noted on the examination and radiograph?
Fluorosis is associated with abnormalities of skeletal and dental tissue. Skeletal fluorosis causes osteosclerosis of the axial skeleton, periosteal new bone formation, ligamentous and tendinous ossification, and osteophyte formation. Dental fluorosis causes a yellow/brown discoloration of the teeth with horizontal streaking (mottling), pitting, and chipping.11 Fluorosis is well-described in regions where water fluoride concentrations are high due to industrial exposure; from consumption of fluorinated wine or chronic overconsumption of tea (especially green or black tea); or from fluoridated toothpaste.12-14 More recently, fluorosis has been described in patients treated for an extended duration of time with voriconazole, a fluorinated antifungal agent.15 Unlike other hydrocarbon products, fluorinated hydrocarbons such as 1,1-DFE can significantly increase systemic fluoride concentrations with excessive use. Rapid skeletal fluorosis is not well described, but has been reported after chronic abuse of fluorinated hydrocarbons.16
How is fluorosis diagnosed and managed?
The lack of rapid laboratory testing available for serum, urine, and bone fluoride concentrations makes the initial diagnosis of fluorosis a clinical one. Imaging studies are generally highly suggestive of fluorosis and can be used to support the diagnosis. A dual energy X-ray absorptiometry scan of the spine, hip, femur, and distal portions of the radii can reveal elevated T-scores consistent with osteosclerosis.14 These findings, in conjunction with bone or joint pain, reduced range of motion, or kyphosis, should prompt clinicians to conduct further testing—even without a confirmed fluoride source. A serum fluoride (reference range, 0.2-3.2 mg/L) and 24-hour urine fluoride (reference range, 0.2-3.2 mg/dL) and creatinine evaluation can be used to diagnose fluorosis. However, a bone biopsy with quantitative bone ash fluoride analysis remains the gold standard for the diagnosis of skeletal fluorosis.16 Laboratory evaluation should also include an assessment of electrolytes, specifically calcium, 25-hydroxyvitamin D, and alkaline phosphatase. The differential diagnosis should include hemoglobinopathies, renal osteodystrophy, Paget disease, hypothyroidism, and skeletal metastases.16
Treatment of fluorosis is largely symptomatic and supportive, with identification and discontinuation of the fluoride source. Patients should be referred to an orthopedist for evaluation and management as needed. Evaluation by an endocrinologist should also be considered because patients may have chronic vitamin D and calcium deficiencies as a result of systemic fluorosis.
Case Conclusion
The patient’s laboratory assessment was notable for the following: alkaline phosphatase, 624 U/L (reference range, 44-147 IU/L); vitamin D, 10 ng/mL (reference range, 20-40 ng/mL); serum fluoride, 0.3 mg/L (reference range, 0.2-3.2 mg/L); urine fluoride, 52 mg/dL (0.2-3.2 mg/dL); and urine creatinine, 1 g/L (reference range, 0.3-3 g/L). Imaging studies noted periosteal bone formation on the lateral epicondyle of the distal right humerus, as well as similar osseous abnormalities in other locations. A bone biopsy was scheduled. The patient was treated with oral vitamin D and educated about the importance of discontinuing the huffing of all hydrocarbons.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.
1. Williams JF, Storck M; American Academy of Pediatrics Committee on Substance Abuse; American Academy of Pediatrics Committee on Native American Child Health. Inhalant abuse. Pediatrics. 2007;119(5):1009-1017.
2. Wu LT, Pilowsky DJ, Schlenger WE. Inhalant abuse and dependence among adolescents in the United States. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1206-1214.
3. Avella J, Kunaparaju N, Kumar S, Lehrer M, Zito SW, Barletta M. Uptake and distribution of the abused inhalant 1,1-difluoroethane in the rat. J Anal Toxicol. 2010;34(7):381-388.
4. Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: A review. Clin Toxicol (Phila). 2014;52(5):479-489.
5. Duncan JR, Lawrence AJ. Conventional concepts and new perspectives for understanding the addictive properties of inhalants. J Pharmacol Sci. 2013;122(4):237-243.
6. Sakai K, Maruyama-Maebashi K, Takatsu A, et al. Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int. 2011;206(1-3):e58-e61.
7. Himmel HM. Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol. 2008;38(9):773-803.
8. Avella J, Wilson JC, Lehrer M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27(1):58-60.
9. Nelson LS. Toxicologic myocardial sensitization. J Toxicol Clin Toxicol. 2002;40(7):867-879.
10. Mortiz F, de La Chapelle A, Bauer F, Leroy JP, Goullé JP, Bonmarchand G. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning. Intensive Care Med. 2000;26(2):256.
11. Majumdar KK. Health impact of supplying safe drinking water containing fluoride below permissible level on flourosis patients in a fluoride-endemic rural area of West Bengal. Indian J Public Health. 2011;55(4):303-308.
12. Kakumanu N, Rao SD. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N Engl J Med 2013;368(12):1140.
13. Soriano M, Manchón F. Radiological aspects of a new type of bone fluorosis, periostitis deformans. Radiology 1966;87(6):1089-1094.
14. Tamer MN, Kale Köroğlu B, Arslan C, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373(1):43-48.
15. Bucknor MD, Gross AJ, Link TM. Voriconazole-induced periostitis in two post-transplant patients. J Radiol Case Rep. 2013;7(8):10-17.
16. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connector. 2014;4(4):e108.