User login
Psychiatric consultations in long-term care: An evidence-based practical guide
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
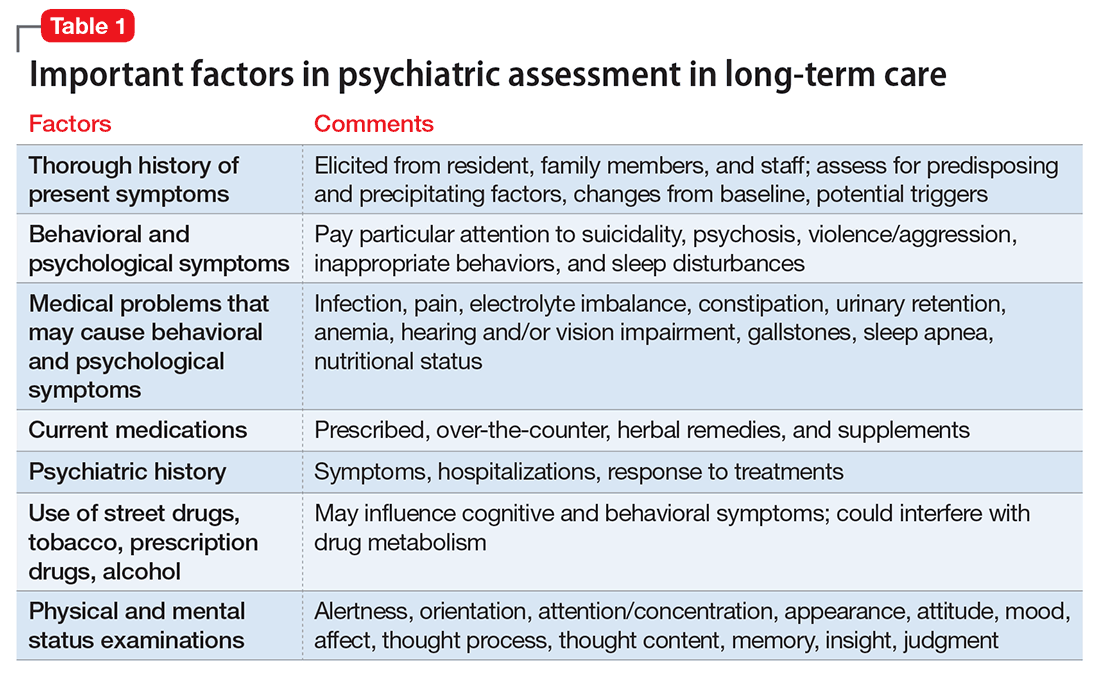
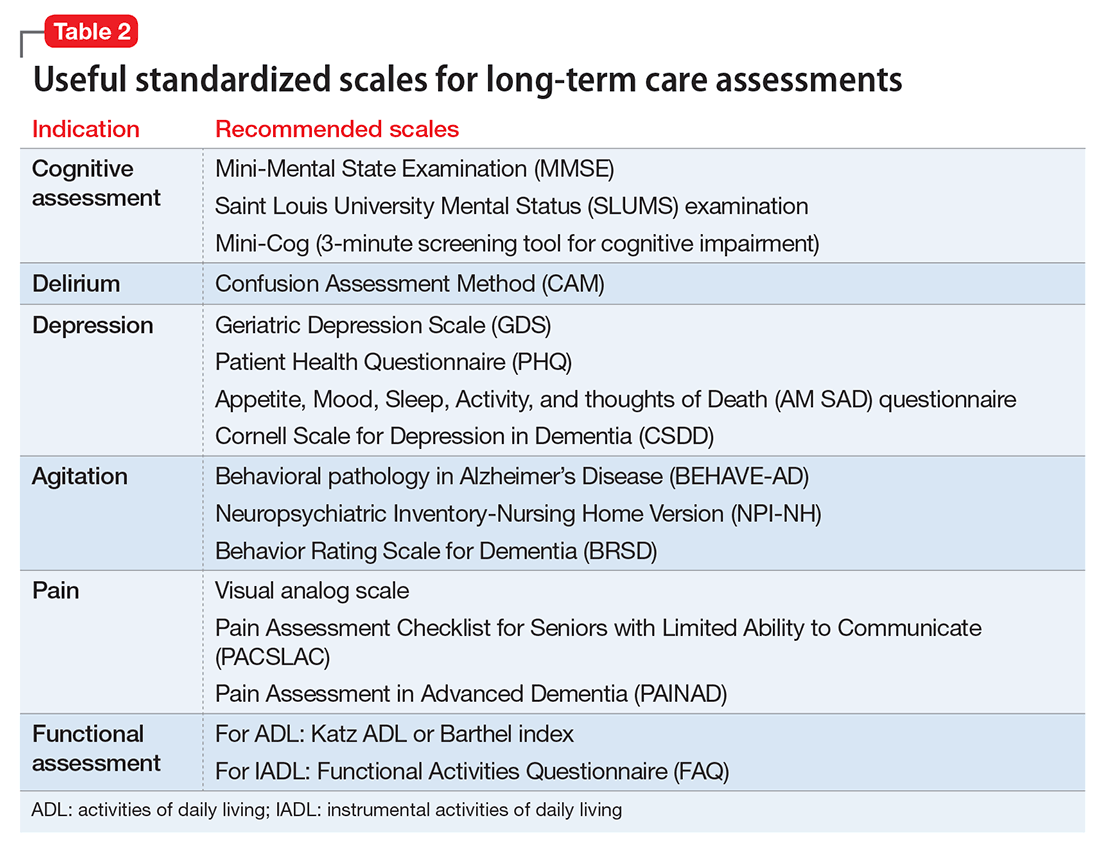
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
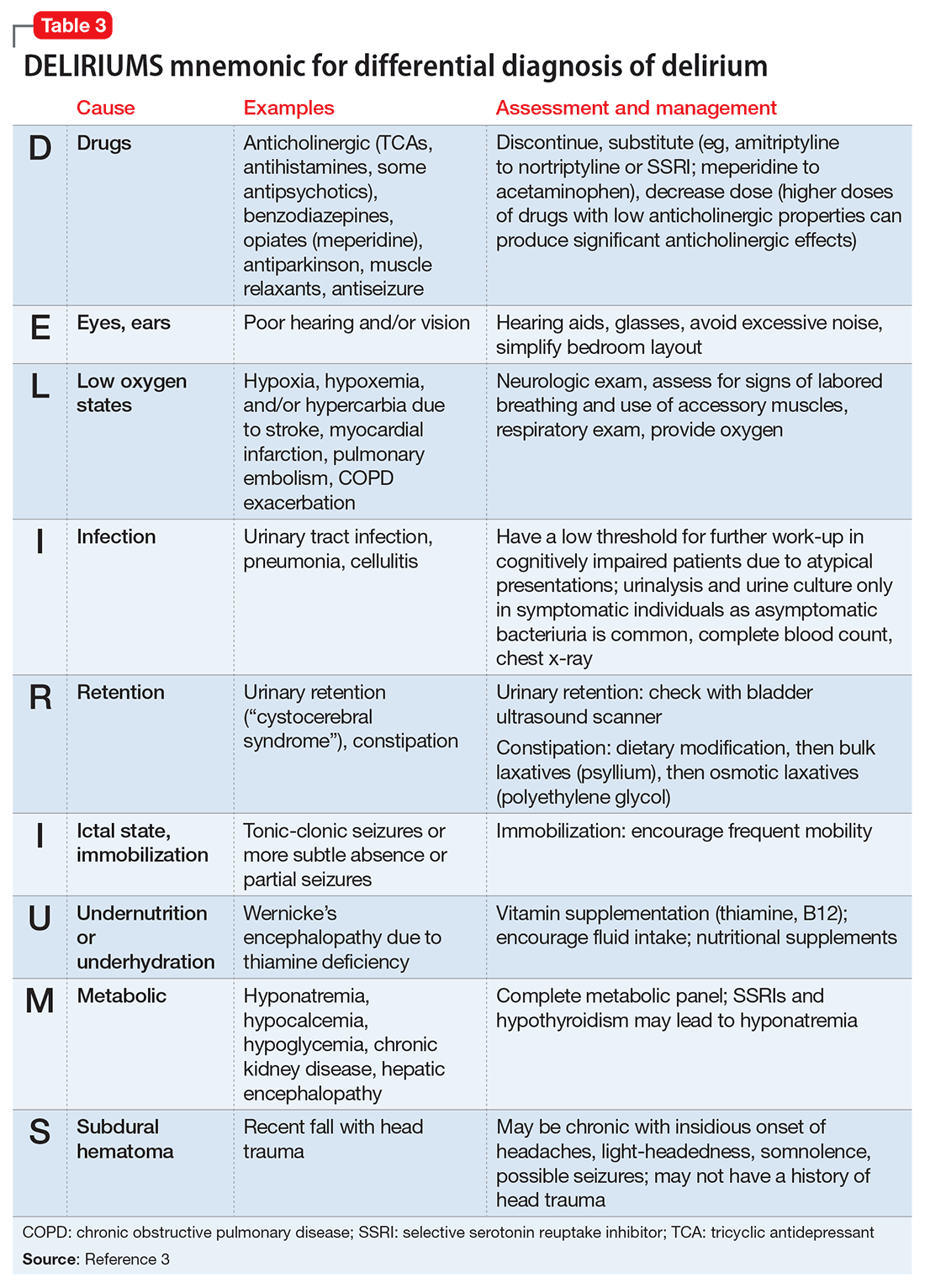
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
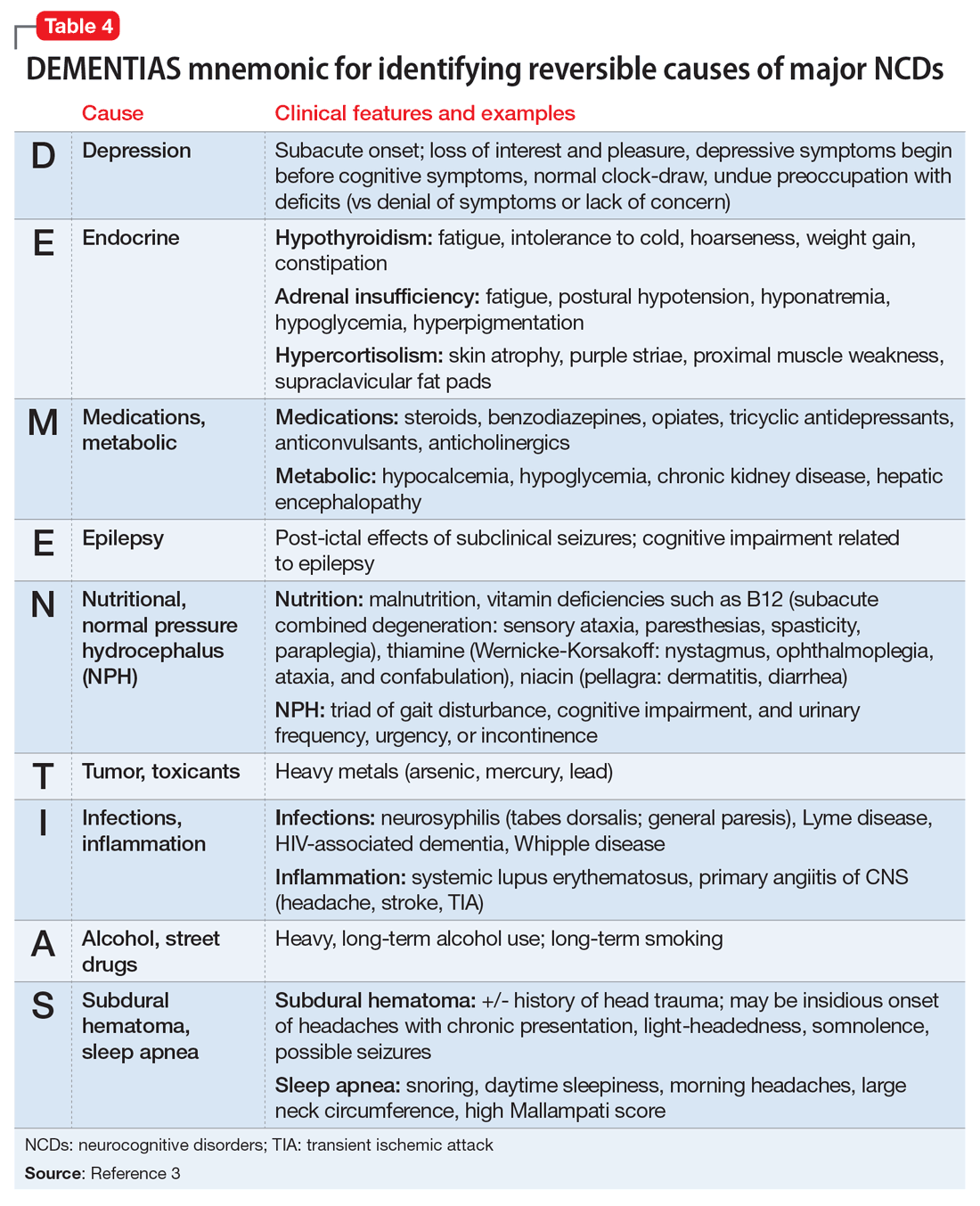
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.