User login
Cesarean Delivery Rates Among Nulliparous Women Are Lower After Guidance
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Postpartum Glycemic Screening Rates Are Low for Women With GDM History
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
FROM OBSTETRICS & GYNECOLOGY
Cesarean delivery rates among nulliparous women are lower after guidance
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Adoption of the 2014 consensus guidelines for the prevention of primary cesarean delivery significantly decreased the C-section rate at one medical center.
Major finding: After guideline adoption, the cesarean delivery among patients who were induced or augmented fell from 35.5% to 24.5%.
Data sources: A before-after retrospective cohort study of 567 patients at a single academic center between Sept. 13, 2013 and Sept. 28, 2014.
Disclosures: No funding source was reported and the researchers reported having no potential conflicts of interest.
Obesity in healthy women linked to poor pregnancy and neonatal outcomes
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: For women without chronic diseases, prepregnancy obesity increases risks for adverse pregnancy and neonatal outcomes.
Major finding: The relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Data sources: Maternal and neonatal medical records from 112,309 singleton deliveries from 2002-2008.
Disclosures: The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Postpartum glycemic screening rates are low for women with GDM history
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Glycemic screening rates for women who had gestational diabetes mellitus are generally low and vary based on race, geography, and medication treatment in pregnancy.
Major finding: More than three-quarters of women with a history of gestational diabetes mellitus did not receive a glycemic screen in their first year postpartum.
Data sources: A retrospective analysis of medical records from women enrolled in a large, U.S. commercial health plan from 2000-2012.
Disclosures: The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Maternal vaccination against pertussis can protect premature infants
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
FROM PEDIATRICS
Maternal vaccination against pertussis can protect premature infants
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
FROM PEDIATRICS
Key clinical point: Vaccination of pregnant women early in their third trimester may protect premature infants against pertussis.
Major finding: At 2 months after birth, infants born to vaccinated mothers displayed higher antibody concentrations to vaccine antigens than did infants born to unvaccinated mothers.
Data source: Premature infants born to mothers that did or did not receive Repevax from 28 weeks of pregnancy.
Disclosures: Pfizer Ltd. and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
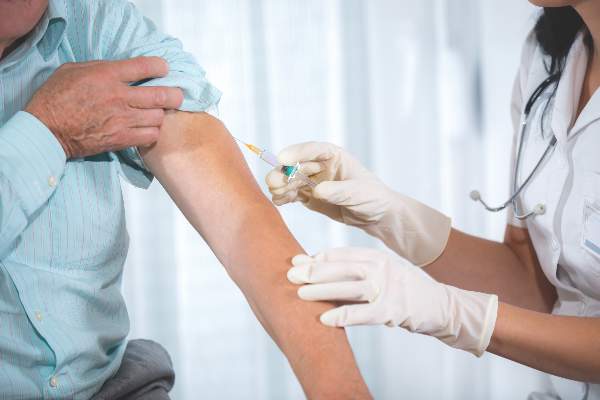
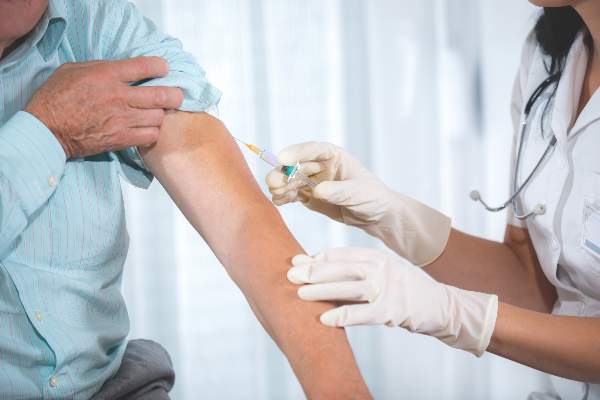
Time of day matters for flu vaccine administration in older adults
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
Time of day matters for flu vaccine administration in older adults
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
Key clinical point: Vaccinating older adults in the morning may improve protection from the influenza virus.
Major finding: Antibody responses to two of three influenza strains were higher when vaccinations were administered in the morning.
Data sources: Participants aged 65 years or older, recruited from 24 Primary Care General Practices within the West Midlands (England), vaccinated in the morning or afternoon between 2011 and 2013.
Disclosures: The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
Ontario’s infant rotavirus immunization program found effective
A publicly-funded rotavirus (RV) immunization program designed to prevent hospitalizations and emergency department (ED) visits for RV-specific acute gastroenteritis (AGE) has shown remarkable improvements in direct and indirect effects 1.5 years after implementation, according to the results of a study published in the PLoS ONE.
“On August 8, 2011, Ontario became one of the first Canadian provinces to implement a universal, publicly-funded RV immunization program, using Rotarix vaccine at 2 and 4 months of age,” noted Dr. Sarah E. Wilson of Public Health Ontario in Toronto and the University of Toronto, and her colleagues. The program was subsequently implemented across 10 of 13 Canadian provinces and territories.
Dr. Wilson and colleagues conducted a retrospective longitudinal population-based cohort study examining health care utilization for AGE between the period of Aug. 1, 2005, and March 31, 2013, as identified from individual-level hospitalizations and ED visits present in the Discharge Abstract Database of the Canadian Institutes for Health Information and the National Ambulatory Care Reporting System, respectively. Furthermore, the study was divided into preprogram (Aug. 1, 2005-July 31, 2011) and a public program (Aug. 1, 2011-March 31, 2013) time periods (PLoS ONE 2016 May 11. doi: 10.1371/journal.pone.0154340).
Study results revealed that infants younger than 1 year of age showed the greatest reduction in the rate of RV-AGE hospitalizations after adjustment for secular trends and seasonality during the public program period (79%, rate ratio 0.21). Children 12-23 months of age (RR 0.27), 24-35 months of age (RR 0.48), 3-4 years of age (RR 0.31), and 5-19 years of age (RR 0.25) also showed significant reductions in RV-AGE hospitalizations. Significant decreases indicative of direct and indirect effects of immunization after adjustment for age, secular trends, and seasonality during the public program period also were demonstrated by a 68% reduction in RV-AGE ED visits (RR 0.32) and a 10% reduction in overall AGE ED visits (RR 0.90).
Regarding the greater implications of their study results, Dr. Wilson and colleagues suggested that their findings should help inform vaccine decision makers in regions without publicly funded RV programs as part of their routine immunization schedules.
Public Health Ontario and the Ontario Ministry of Health and Long-Term Care funded the study. The authors reported no conflicts of interest.
A publicly-funded rotavirus (RV) immunization program designed to prevent hospitalizations and emergency department (ED) visits for RV-specific acute gastroenteritis (AGE) has shown remarkable improvements in direct and indirect effects 1.5 years after implementation, according to the results of a study published in the PLoS ONE.
“On August 8, 2011, Ontario became one of the first Canadian provinces to implement a universal, publicly-funded RV immunization program, using Rotarix vaccine at 2 and 4 months of age,” noted Dr. Sarah E. Wilson of Public Health Ontario in Toronto and the University of Toronto, and her colleagues. The program was subsequently implemented across 10 of 13 Canadian provinces and territories.
Dr. Wilson and colleagues conducted a retrospective longitudinal population-based cohort study examining health care utilization for AGE between the period of Aug. 1, 2005, and March 31, 2013, as identified from individual-level hospitalizations and ED visits present in the Discharge Abstract Database of the Canadian Institutes for Health Information and the National Ambulatory Care Reporting System, respectively. Furthermore, the study was divided into preprogram (Aug. 1, 2005-July 31, 2011) and a public program (Aug. 1, 2011-March 31, 2013) time periods (PLoS ONE 2016 May 11. doi: 10.1371/journal.pone.0154340).
Study results revealed that infants younger than 1 year of age showed the greatest reduction in the rate of RV-AGE hospitalizations after adjustment for secular trends and seasonality during the public program period (79%, rate ratio 0.21). Children 12-23 months of age (RR 0.27), 24-35 months of age (RR 0.48), 3-4 years of age (RR 0.31), and 5-19 years of age (RR 0.25) also showed significant reductions in RV-AGE hospitalizations. Significant decreases indicative of direct and indirect effects of immunization after adjustment for age, secular trends, and seasonality during the public program period also were demonstrated by a 68% reduction in RV-AGE ED visits (RR 0.32) and a 10% reduction in overall AGE ED visits (RR 0.90).
Regarding the greater implications of their study results, Dr. Wilson and colleagues suggested that their findings should help inform vaccine decision makers in regions without publicly funded RV programs as part of their routine immunization schedules.
Public Health Ontario and the Ontario Ministry of Health and Long-Term Care funded the study. The authors reported no conflicts of interest.
A publicly-funded rotavirus (RV) immunization program designed to prevent hospitalizations and emergency department (ED) visits for RV-specific acute gastroenteritis (AGE) has shown remarkable improvements in direct and indirect effects 1.5 years after implementation, according to the results of a study published in the PLoS ONE.
“On August 8, 2011, Ontario became one of the first Canadian provinces to implement a universal, publicly-funded RV immunization program, using Rotarix vaccine at 2 and 4 months of age,” noted Dr. Sarah E. Wilson of Public Health Ontario in Toronto and the University of Toronto, and her colleagues. The program was subsequently implemented across 10 of 13 Canadian provinces and territories.
Dr. Wilson and colleagues conducted a retrospective longitudinal population-based cohort study examining health care utilization for AGE between the period of Aug. 1, 2005, and March 31, 2013, as identified from individual-level hospitalizations and ED visits present in the Discharge Abstract Database of the Canadian Institutes for Health Information and the National Ambulatory Care Reporting System, respectively. Furthermore, the study was divided into preprogram (Aug. 1, 2005-July 31, 2011) and a public program (Aug. 1, 2011-March 31, 2013) time periods (PLoS ONE 2016 May 11. doi: 10.1371/journal.pone.0154340).
Study results revealed that infants younger than 1 year of age showed the greatest reduction in the rate of RV-AGE hospitalizations after adjustment for secular trends and seasonality during the public program period (79%, rate ratio 0.21). Children 12-23 months of age (RR 0.27), 24-35 months of age (RR 0.48), 3-4 years of age (RR 0.31), and 5-19 years of age (RR 0.25) also showed significant reductions in RV-AGE hospitalizations. Significant decreases indicative of direct and indirect effects of immunization after adjustment for age, secular trends, and seasonality during the public program period also were demonstrated by a 68% reduction in RV-AGE ED visits (RR 0.32) and a 10% reduction in overall AGE ED visits (RR 0.90).
Regarding the greater implications of their study results, Dr. Wilson and colleagues suggested that their findings should help inform vaccine decision makers in regions without publicly funded RV programs as part of their routine immunization schedules.
Public Health Ontario and the Ontario Ministry of Health and Long-Term Care funded the study. The authors reported no conflicts of interest.
FROM PLOS ONE
Key clinical point: A publicly funded rotavirus immunization program was successful in preventing hospitalizations and ED visits for RV-specific acute gastroenteritis at the population level.
Major finding: Implementation of the program resulted in significantly increased median age at rotavirus-specific acute gastroenteritis hospitalization and significantly reduced rotavirus-specific acute gastroenteritis hospitalizations and ED visits.
Data sources: Discharge Abstract Database of the Canadian Institutes for Health Information and the National Ambulatory Care Reporting System.
Disclosures: Public Health Ontario and the Ontario Ministry of Health and Long-Term Care funded the study. The authors reported no conflicts of interest.