Article
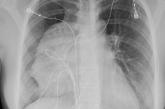
18-year-old woman • chest pain • shortness of breath • electrocardiogram abnormality • Dx?
- Author:
- Cassie Tran, MD
- William Haas, MD
- William Terrill, MD
- Don Nguyen, MD
► Chest pain
► Shortness of breath
► Electrocardiogram abnormality
Article
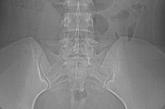
Pelvic pain
- Author:
- William Terrill, MD
- Cassie Tran, MD
- Don Nguyen, MD, MHA
When a complete metabolic panel offered no clues, we turned to imaging. And that’s when we had our diagnosis.
