User login
Does urodynamic testing before surgery for stress incontinence improve outcomes?
URINARY PROBLEMS?
CLICK HERE to access 8 other articles about treating urinary incontinence and urinary tract infections, published in OBG Management in 2012.
Nager and colleagues conducted their trial to determine whether urodynamic testing is necessary before midurethral sling surgery for simple, “garden-variety” stress urinary incontinence (SUI). In their trial, 630 women were randomly assigned to undergo office evaluation with urodynamic tests (n = 315) or office evaluation only (n = 315). Surgical treatment was successful in 76.9% of women in the urodynamic-testing group and in 77.2% of women in the office evaluation-only group (95% confidence interval [CI], –7.5 to 6.9) as long as 1 year after surgery. There were no significant differences between groups in quality of life, patient satisfaction, and other secondary measures of incontinence severity. Investigators concluded that preoperative office evaluation (positive stress test, urethral hypermobility, normal postvoid residual, and negative urinalysis) is not inferior to urodynamic testing.
Should these findings come as a surprise to pelvic surgeons? Retropubic and transobturator slings are associated with very low morbidity and high efficacy. Studies already have demonstrated that, in simple SUI, these slings produce similar outcomes.1 So there is some consensus that urodynamic testing may be unnecessary prior to placement of a midurethral sling for simple SUI. This study lends credence to that notion.
Outcome measures: Focused enough?
The primary outcome measures used in this trial were not designed primarily to assess the severity of SUI. Investigators employed the Urogenital Distress Inventory, which focuses mostly on irritative bladder symptoms and includes only one question about the presence and impact of SUI. They also utilized the Patient Global Impression of Improvement, a global scale related to incontinence in general. Other tests—such as standardized stress tests, pad tests, and bladder diaries—are more specific in the evaluation of SUI severity. Nager and colleagues used these measures themselves in an earlier exploration of the role of urodynamics in predicting sling failure.2
Moreover, in the current study, urodynamic testing did not include a measurement of urethral closure pressure, which has been strongly correlated with success after Burch colposuspension and retropubic and transobturator sling procedures. This test would have added valuable data.
Another shortcoming was the lack of uniformity in the sling procedures that were selected. Most experts believe that retropubic and transobturator slings have unique mechanistic characteristics that do not allow them to be interchangeable. Investigators should have selected a single sling approach.
Is a 77% success rate acceptable to most patients?
SUI represents a continuum of disease severity, and various studies have demonstrated a higher success rate for retropubic slings than for the transobturator approach when sphincteric function is impaired. At our center, we utilize urodynamic parameters to identify women who may achieve a higher continence rate with a retropubic sling.3
Retropubic slings are not appropriate for all patients with SUI. Although the risk of serious complications, such as retropubic hematoma or bowel perforation, is very low, complications sometimes do occur and are related to surgical volumes. For this reason, transobturator slings, which carry minimal associated risks, play a key role in the management of garden-variety SUI.
Indications for multichannel urodynamic testing
|
Don’t throw away your urodynamic equipment just yet. Many patients have complex incontinence symptoms or other indications for urodynamic testing (TABLE). In addition, a clear understanding of urgency symptoms and voiding dysfunction can be very useful during preoperative counseling of patients who are scheduled to undergo sling surgery. Significant urgency is likely to persist postoperatively, and voiding difficulties may worsen after a sling procedure, especially if a retropubic sling is used.
G. WILLY DAVILA, MD
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
1. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
2. Nager CW, Siris L, Litman HJ, et al. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186:597-603.
3. Guerette NL, Bena JF, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J. 2008;19(1):97-102.
URINARY PROBLEMS?
CLICK HERE to access 8 other articles about treating urinary incontinence and urinary tract infections, published in OBG Management in 2012.
Nager and colleagues conducted their trial to determine whether urodynamic testing is necessary before midurethral sling surgery for simple, “garden-variety” stress urinary incontinence (SUI). In their trial, 630 women were randomly assigned to undergo office evaluation with urodynamic tests (n = 315) or office evaluation only (n = 315). Surgical treatment was successful in 76.9% of women in the urodynamic-testing group and in 77.2% of women in the office evaluation-only group (95% confidence interval [CI], –7.5 to 6.9) as long as 1 year after surgery. There were no significant differences between groups in quality of life, patient satisfaction, and other secondary measures of incontinence severity. Investigators concluded that preoperative office evaluation (positive stress test, urethral hypermobility, normal postvoid residual, and negative urinalysis) is not inferior to urodynamic testing.
Should these findings come as a surprise to pelvic surgeons? Retropubic and transobturator slings are associated with very low morbidity and high efficacy. Studies already have demonstrated that, in simple SUI, these slings produce similar outcomes.1 So there is some consensus that urodynamic testing may be unnecessary prior to placement of a midurethral sling for simple SUI. This study lends credence to that notion.
Outcome measures: Focused enough?
The primary outcome measures used in this trial were not designed primarily to assess the severity of SUI. Investigators employed the Urogenital Distress Inventory, which focuses mostly on irritative bladder symptoms and includes only one question about the presence and impact of SUI. They also utilized the Patient Global Impression of Improvement, a global scale related to incontinence in general. Other tests—such as standardized stress tests, pad tests, and bladder diaries—are more specific in the evaluation of SUI severity. Nager and colleagues used these measures themselves in an earlier exploration of the role of urodynamics in predicting sling failure.2
Moreover, in the current study, urodynamic testing did not include a measurement of urethral closure pressure, which has been strongly correlated with success after Burch colposuspension and retropubic and transobturator sling procedures. This test would have added valuable data.
Another shortcoming was the lack of uniformity in the sling procedures that were selected. Most experts believe that retropubic and transobturator slings have unique mechanistic characteristics that do not allow them to be interchangeable. Investigators should have selected a single sling approach.
Is a 77% success rate acceptable to most patients?
SUI represents a continuum of disease severity, and various studies have demonstrated a higher success rate for retropubic slings than for the transobturator approach when sphincteric function is impaired. At our center, we utilize urodynamic parameters to identify women who may achieve a higher continence rate with a retropubic sling.3
Retropubic slings are not appropriate for all patients with SUI. Although the risk of serious complications, such as retropubic hematoma or bowel perforation, is very low, complications sometimes do occur and are related to surgical volumes. For this reason, transobturator slings, which carry minimal associated risks, play a key role in the management of garden-variety SUI.
Indications for multichannel urodynamic testing
|
Don’t throw away your urodynamic equipment just yet. Many patients have complex incontinence symptoms or other indications for urodynamic testing (TABLE). In addition, a clear understanding of urgency symptoms and voiding dysfunction can be very useful during preoperative counseling of patients who are scheduled to undergo sling surgery. Significant urgency is likely to persist postoperatively, and voiding difficulties may worsen after a sling procedure, especially if a retropubic sling is used.
G. WILLY DAVILA, MD
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
URINARY PROBLEMS?
CLICK HERE to access 8 other articles about treating urinary incontinence and urinary tract infections, published in OBG Management in 2012.
Nager and colleagues conducted their trial to determine whether urodynamic testing is necessary before midurethral sling surgery for simple, “garden-variety” stress urinary incontinence (SUI). In their trial, 630 women were randomly assigned to undergo office evaluation with urodynamic tests (n = 315) or office evaluation only (n = 315). Surgical treatment was successful in 76.9% of women in the urodynamic-testing group and in 77.2% of women in the office evaluation-only group (95% confidence interval [CI], –7.5 to 6.9) as long as 1 year after surgery. There were no significant differences between groups in quality of life, patient satisfaction, and other secondary measures of incontinence severity. Investigators concluded that preoperative office evaluation (positive stress test, urethral hypermobility, normal postvoid residual, and negative urinalysis) is not inferior to urodynamic testing.
Should these findings come as a surprise to pelvic surgeons? Retropubic and transobturator slings are associated with very low morbidity and high efficacy. Studies already have demonstrated that, in simple SUI, these slings produce similar outcomes.1 So there is some consensus that urodynamic testing may be unnecessary prior to placement of a midurethral sling for simple SUI. This study lends credence to that notion.
Outcome measures: Focused enough?
The primary outcome measures used in this trial were not designed primarily to assess the severity of SUI. Investigators employed the Urogenital Distress Inventory, which focuses mostly on irritative bladder symptoms and includes only one question about the presence and impact of SUI. They also utilized the Patient Global Impression of Improvement, a global scale related to incontinence in general. Other tests—such as standardized stress tests, pad tests, and bladder diaries—are more specific in the evaluation of SUI severity. Nager and colleagues used these measures themselves in an earlier exploration of the role of urodynamics in predicting sling failure.2
Moreover, in the current study, urodynamic testing did not include a measurement of urethral closure pressure, which has been strongly correlated with success after Burch colposuspension and retropubic and transobturator sling procedures. This test would have added valuable data.
Another shortcoming was the lack of uniformity in the sling procedures that were selected. Most experts believe that retropubic and transobturator slings have unique mechanistic characteristics that do not allow them to be interchangeable. Investigators should have selected a single sling approach.
Is a 77% success rate acceptable to most patients?
SUI represents a continuum of disease severity, and various studies have demonstrated a higher success rate for retropubic slings than for the transobturator approach when sphincteric function is impaired. At our center, we utilize urodynamic parameters to identify women who may achieve a higher continence rate with a retropubic sling.3
Retropubic slings are not appropriate for all patients with SUI. Although the risk of serious complications, such as retropubic hematoma or bowel perforation, is very low, complications sometimes do occur and are related to surgical volumes. For this reason, transobturator slings, which carry minimal associated risks, play a key role in the management of garden-variety SUI.
Indications for multichannel urodynamic testing
|
Don’t throw away your urodynamic equipment just yet. Many patients have complex incontinence symptoms or other indications for urodynamic testing (TABLE). In addition, a clear understanding of urgency symptoms and voiding dysfunction can be very useful during preoperative counseling of patients who are scheduled to undergo sling surgery. Significant urgency is likely to persist postoperatively, and voiding difficulties may worsen after a sling procedure, especially if a retropubic sling is used.
G. WILLY DAVILA, MD
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
1. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
2. Nager CW, Siris L, Litman HJ, et al. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186:597-603.
3. Guerette NL, Bena JF, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J. 2008;19(1):97-102.
1. Barber MD, Kleeman S, Karram MM, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence. Obstet Gynecol. 2008;111(3):611-621.
2. Nager CW, Siris L, Litman HJ, et al. Baseline urodynamic predictors of treatment failure 1 year after midurethral sling surgery. J Urol. 2011;186:597-603.
3. Guerette NL, Bena JF, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J. 2008;19(1):97-102.
Take this simplified approach to correcting exposure of vaginal mesh
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
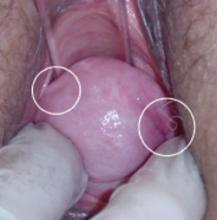
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?
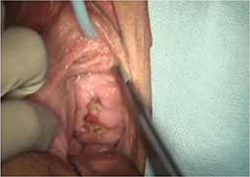
FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
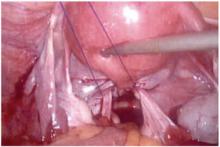
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).
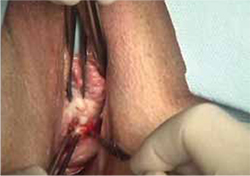
FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.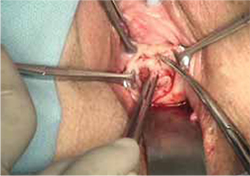
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.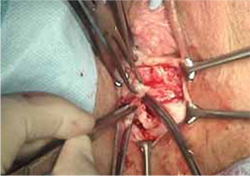
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!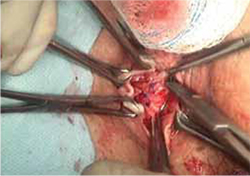
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.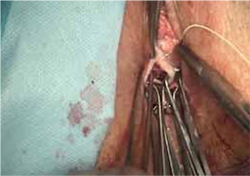
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
Pessary and pelvic floor exercises for incontinence—are two better than one?
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
Q Can a questionnaire differentiate urge and stress incontinence?
The 3IQ is therefore acceptable for use by primary care physicians, especially when treatment will be noninvasive.
Expert Commentary
When a woman complains of urinary incontinence, the clinician’s first objective is determining which type of incontinence she has so therapy can be appropriately targeted. This is not as straightforward as might be expected. The symptoms of urge and stress incontinence overlap significantly, necessitating simple or complex urodynamics.
Simple conservative treatments such as timed voiding and Kegel exercises may benefit all incontinent women. However, other treatments, such as anticholinergic drugs for urge incontinence or the new transobturator sling procedures for stress incontinence, require a more precise diagnosis. Thus was born the 3IQ.
1. During the last 3 months, have you leaked urine?
□ Yes
□ No
If no, no need to proceed
2. During the last 3 months, did you leak urine: (Check all that apply)
□ a. When you were performing a physical activity such as coughing, sneezing, lifting, or exercise?
□ b. When you had the urge or need to empty your bladder, but could not get to the toilet fast enough?
□ c. Without physical activity and without a sense of urgency?
3. During the last 3 months, did you leak urine most often: (Check only one)
□ a. When you were performing a physical activity?
□ b. When you had the urge or the feeling that you needed to empty your bladder?
□ c. Without physical activity and without a sense of urgency?
□ d. About equally as often with physical activity as with a sense of urgency?
Score by response to question 3:
a=stress or stress-dominant
b=urge or urge-dominant
c=other causes
d=mixed
Adapted from Brown JS, et al
How the 3IQ assesses symptoms
The 3IQ was completed by 301 women with untreated incontinence and was compared with a “gold standard” evaluation that included a history, physical examination (including neurologic evaluation), pelvic exam, cough stress test, measurement of postvoid residual, and review of a 3-day voiding diary.
Investigators did not evaluate each question individually, which is unfortunate, as it would be interesting to know whether a single question has similar value in distinguishing stress and urge incontinence.
All the women attended tertiary continence centers. It would be helpful to repeat this study in a primary care setting where women may have less severe symptoms and be less likely to undergo further evaluation.
Despite limited utility to ObGyns, the study is good news
The 3IQ appears to be particularly useful for selecting noninvasive therapy for women with urge incontinence. For surgeons who perform minimally invasive sling procedures for stress incontinence, the low specificity of this test renders it inappropriate. Thus, the 3IQ may be more useful for a family practitioner or internist than for the ObGyn who is also a surgeon.
The fact that this study was published in Annals of Internal Medicine makes it clear that primary care physicians are interested in the care of women with urinary incontinence. ObGyns should be happy about this, as studies like this one will increase awareness of the frequency of urinary incontinence and lead to further referrals to ObGyns for more extensive management.
The 3IQ is therefore acceptable for use by primary care physicians, especially when treatment will be noninvasive.
Expert Commentary
When a woman complains of urinary incontinence, the clinician’s first objective is determining which type of incontinence she has so therapy can be appropriately targeted. This is not as straightforward as might be expected. The symptoms of urge and stress incontinence overlap significantly, necessitating simple or complex urodynamics.
Simple conservative treatments such as timed voiding and Kegel exercises may benefit all incontinent women. However, other treatments, such as anticholinergic drugs for urge incontinence or the new transobturator sling procedures for stress incontinence, require a more precise diagnosis. Thus was born the 3IQ.
1. During the last 3 months, have you leaked urine?
□ Yes
□ No
If no, no need to proceed
2. During the last 3 months, did you leak urine: (Check all that apply)
□ a. When you were performing a physical activity such as coughing, sneezing, lifting, or exercise?
□ b. When you had the urge or need to empty your bladder, but could not get to the toilet fast enough?
□ c. Without physical activity and without a sense of urgency?
3. During the last 3 months, did you leak urine most often: (Check only one)
□ a. When you were performing a physical activity?
□ b. When you had the urge or the feeling that you needed to empty your bladder?
□ c. Without physical activity and without a sense of urgency?
□ d. About equally as often with physical activity as with a sense of urgency?
Score by response to question 3:
a=stress or stress-dominant
b=urge or urge-dominant
c=other causes
d=mixed
Adapted from Brown JS, et al
How the 3IQ assesses symptoms
The 3IQ was completed by 301 women with untreated incontinence and was compared with a “gold standard” evaluation that included a history, physical examination (including neurologic evaluation), pelvic exam, cough stress test, measurement of postvoid residual, and review of a 3-day voiding diary.
Investigators did not evaluate each question individually, which is unfortunate, as it would be interesting to know whether a single question has similar value in distinguishing stress and urge incontinence.
All the women attended tertiary continence centers. It would be helpful to repeat this study in a primary care setting where women may have less severe symptoms and be less likely to undergo further evaluation.
Despite limited utility to ObGyns, the study is good news
The 3IQ appears to be particularly useful for selecting noninvasive therapy for women with urge incontinence. For surgeons who perform minimally invasive sling procedures for stress incontinence, the low specificity of this test renders it inappropriate. Thus, the 3IQ may be more useful for a family practitioner or internist than for the ObGyn who is also a surgeon.
The fact that this study was published in Annals of Internal Medicine makes it clear that primary care physicians are interested in the care of women with urinary incontinence. ObGyns should be happy about this, as studies like this one will increase awareness of the frequency of urinary incontinence and lead to further referrals to ObGyns for more extensive management.
The 3IQ is therefore acceptable for use by primary care physicians, especially when treatment will be noninvasive.
Expert Commentary
When a woman complains of urinary incontinence, the clinician’s first objective is determining which type of incontinence she has so therapy can be appropriately targeted. This is not as straightforward as might be expected. The symptoms of urge and stress incontinence overlap significantly, necessitating simple or complex urodynamics.
Simple conservative treatments such as timed voiding and Kegel exercises may benefit all incontinent women. However, other treatments, such as anticholinergic drugs for urge incontinence or the new transobturator sling procedures for stress incontinence, require a more precise diagnosis. Thus was born the 3IQ.
1. During the last 3 months, have you leaked urine?
□ Yes
□ No
If no, no need to proceed
2. During the last 3 months, did you leak urine: (Check all that apply)
□ a. When you were performing a physical activity such as coughing, sneezing, lifting, or exercise?
□ b. When you had the urge or need to empty your bladder, but could not get to the toilet fast enough?
□ c. Without physical activity and without a sense of urgency?
3. During the last 3 months, did you leak urine most often: (Check only one)
□ a. When you were performing a physical activity?
□ b. When you had the urge or the feeling that you needed to empty your bladder?
□ c. Without physical activity and without a sense of urgency?
□ d. About equally as often with physical activity as with a sense of urgency?
Score by response to question 3:
a=stress or stress-dominant
b=urge or urge-dominant
c=other causes
d=mixed
Adapted from Brown JS, et al
How the 3IQ assesses symptoms
The 3IQ was completed by 301 women with untreated incontinence and was compared with a “gold standard” evaluation that included a history, physical examination (including neurologic evaluation), pelvic exam, cough stress test, measurement of postvoid residual, and review of a 3-day voiding diary.
Investigators did not evaluate each question individually, which is unfortunate, as it would be interesting to know whether a single question has similar value in distinguishing stress and urge incontinence.
All the women attended tertiary continence centers. It would be helpful to repeat this study in a primary care setting where women may have less severe symptoms and be less likely to undergo further evaluation.
Despite limited utility to ObGyns, the study is good news
The 3IQ appears to be particularly useful for selecting noninvasive therapy for women with urge incontinence. For surgeons who perform minimally invasive sling procedures for stress incontinence, the low specificity of this test renders it inappropriate. Thus, the 3IQ may be more useful for a family practitioner or internist than for the ObGyn who is also a surgeon.
The fact that this study was published in Annals of Internal Medicine makes it clear that primary care physicians are interested in the care of women with urinary incontinence. ObGyns should be happy about this, as studies like this one will increase awareness of the frequency of urinary incontinence and lead to further referrals to ObGyns for more extensive management.
Choosing the best technique for vaginal vault prolapse
- Look for vault prolapse in any woman who has an advanced degree of vaginal prolapse.
- Goals of surgery: to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function.
- If sexual function is critical to the patient, a sacrocolpopexy should be the primary surgical option.
- Preoperative low-dose estrogen cream is crucial in most postmenopausal women.
Identifying vault prolapse can be difficult in a woman with extensive vaginal prolapse, and operative failure is likely if support to the apex is not restored.
Because this condition is so challenging to identify, many women undergoing anterior and/or posterior colporrhaphy likely have undiagnosed vault prolapse. This may contribute to the 29.2% rate of reoperation in women who undergo pelvic floor reconstructive procedures.1
This article reviews the anatomy of apical support, tells how to identify vaginal vault prolapse during the physical exam, and outlines effective surgical options—both vaginal and abdominal—for its correction. We focus on accurate pelvic assessment as the basis for planning the surgery.
Vaginal stability is fragile
The stability of vaginal anatomy is precarious, since it depends on a series of interrelationships between both dynamic and static structures. When the relationships between the ligaments and fascia at the vaginal apex or vault are impaired, vault prolapse ensues.
Thanks to cadaveric and radiographic studies, our understanding of the complexities of vaginal anatomy has improved considerably; still, the area of vaginal support we least understand is the coalescence of ligaments and fascia at the vaginal apex or vault.
Grade II prolapse, at least, in 64.8%
An analysis of Women’s Health Initiative enrollees with an intact uterus found that 64.8% had at least grade II prolapse (ie, leading edge of prolapse at –1 to +1 cm from the hymen) according to the Pelvic Organ Prolapse Quantification System (POP-Q).2 Approximately 8% of enrollees had a point D (vaginal apex) of greater than –6 cm, suggesting some degree of vault prolapse.
Hysterectomy appears to contribute. The incidence is about 1% at 3 years; 5% at 17 years.3
In the United States, approximately 30,000 vaginal vault repairs were performed in 1999.
Normal support structure
Several support structures coalesce at the vaginal apex. If the cervix is present, it serves as an obvious strong attachment site (FIGURE 1). In hysterectomized women, the structures may lack a strong attachment site, resulting in weakness and prolapse.
FIGURE 1 Vaginal support system
The coalescence of both sets of ligaments forms the uterosacral-cardinal ligament complex at the vaginal apex, which is likely crucial to vault support. Reprinted with permission of The Cleveland Clinic Foundation.
2 sets of ligaments determine support
Uterosacral ligaments—peritoneal and fibromuscular tissue bands extending from the vaginal apex to the sacrum—are the principal support for the vaginal apex, despite their apparent lack of strength.
The role of the cardinal ligaments—which extend laterally from the apex to the pelvic sidewall, adjacent to the ischial spine—is less clear. Since they lie proximal to the ureters, restoring vault support by shortening or reattaching them to the apex is a less attractive option.
The coalescence of these 2 sets of ligaments forms the complex that likely maintains vault support.
In hysterectomized women, locating the attachment of this complex to the vaginal cuff (seen on the exam as apical “dimples”) is key to identifying vault prolapse.
New view of cystoceles, rectoceles
The fibromuscular tissue layer underlying the vaginal epithelium envelops the entire vaginal canal, extending from apex to perineum and from arcus tendineus to arcus tendineus.
As the aponeurosis does for the abdominal wall, the endopelvic fascia maintains integrity of the anterior and posterior vaginal walls. If the fascial layer detaches from the vaginal apex, a true hernia can develop in the form of an enterocele—anterior or posterior—further weakening vault integrity (FIGURE 2).
Reconstructive surgeons are beginning to view cystoceles and rectoceles as a detachment of the endopelvic fascia from the vaginal apex. Thus, it is critical to restore anterior and posterior vaginal wall fascial integrity from apex to perineum by reattaching the endogenous fascia to the vaginal apex, or by placing a biologic or synthetic graft.
FIGURE 2 Apical defects contribute to vault prolapse
Vault prolapse is often associated with defects of the apical fascia, represented here by dark lines, which must be addressed during vault reconstruction. Reprinted with permission of The Cleveland Clinic Foundation.
Specific technique, tools to help identify prolapse
Any patient with an advanced degree of vaginal prolapse should be assessed for vault prolapse using a careful, structured pelvic exam. In many cases, this can be difficult, even if the uterus is present.
Necessary tools include a bivalved speculum and a right-angle retractor, or the posterior blade of another gynecologic speculum.
When the uterus is present
An exteriorized cervix does not necessarily mean vault prolapse; this may occur with substantial cervical hypertrophy, while the apex remains well supported (FIGURE 3).
Exam technique. Place the right-angle speculum blade in the posterior fornix, inserting it to its full extent, and ask the patient to perform a Valsalva maneuver. If vault prolapse is present, the uterus will descend further as the speculum is slowly removed; reinsertion of the speculum will resuspend the uterus. If the vault is well supported, the cervix will remain in place despite Valsalva efforts.
Assess the degree of vault prolapse during this examination, to determine whether a McCall culdoplasty will restore vault support.
If uterine suspension is performed in a woman with substantial cervical hypertrophy, cervical prolapse may persist, necessitating partial amputation (Manchester procedure).
FIGURE 3 Exteriorized cervix does not necessarily mean vault prolapse
Cervical prolapse may be associated with vault prolapse (left) or simply represent cervical hypertrophy without vault prolapse (right). Reprinted with permission of The Cleveland Clinic Foundation.
In the hysterectomized patient
The goal of physical exam is to identify the apical scar tissue (cuff) resultant from the hysterectomy. In most women, the cuff is visible as a transverse band of tissue firmer than the adjacent vaginal walls. If the woman has extensive prolapse, the tissue is stretched and thus not as obvious.
Exam technique. Use a bivalved speculum to visualize the apex. In women with extensive prolapse, redundant vaginal tissue may impede visualization. Fortunately, the sites of previous attachment of the uterosacral-cardinal ligament complex can usually be identified as “dimples” on either side of the midline at the cuff (FIGURE 4).
Use both right-angle speculum blades, or 1 blade along the anterior vaginal wall and the index and middle fingers of your other hand along the posterior vaginal wall, to identify the dimples. Then place the tip of the speculum between the dimples, elevate the vault while the patient performs a Valsalva effort, and determine the degree of vault prolapse. This can be confirmed by digital exam by identifying the dimples by tact and elevating them to their ipsilateral ischial spines.
FIGURE 4 Identifying the vault in the hysterectomized patient
Posthysterectomy vault prolapse can be identified by looking for “dimples” at the apex, which represent sites of previous uterosacral-cardinal ligament complex attachment. Reprinted with permission of The Cleveland Clinic Foundation.
Which exam findings point to which technique?
The importance of accurate pelvic assessment is impossible to overemphasize. Besides determining the degree and type of prolapse present, the exam enhances surgical planning. Fascial tears or defects are usually identifiable during careful vaginal exam as areas of sudden change in the thickness of the vaginal wall.
By the end of the pelvic exam, we usually have developed a surgical plan for the prolapse repair, pending urodynamic assessment to determine the best anti-incontinence procedure, if necessary.
What are the surgical goals?
Objectives are to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function—without precipitating new support or functional problems.
Abdominal versus vaginal approach
Most surgeons prefer a vaginal approach to pelvic reconstruction. However, this decision should be based on the patient’s individual variables.
If sexual function is critical to the patient, a sacrocolpopexy should be the primary option. Note that age does not always predict the importance of sexual function.
Vaginal length. If the vaginal apex (dimples) reaches the ischial spines with ease, a vaginal procedure should suffice. If it does not reach the spines, or extends far above, an abdominal sacrocolpopexy or obliterative procedure may more be appropriate.
Previous reconstructive procedures. Keep in mind that the area around the sacral promontory, or sacrospinous ligaments, may be difficult or risky to reach due to scarring and fibrosis. This is doubly important in this age of commonplace graft use.
Large paravaginal defects. Vaginal repairs can be technically difficult, and long-term outcomes have not been reported. An abdominal approach is probably better if substantial paravaginal defects are present.
Medical comorbidities. Use a vaginal or obliterative procedure under regional anesthesia if the patient is medically delicate or elderly.
Tissue quality usually improves with preoperative local estrogen, but large fascial defects adjacent to the cuff or perineum may require graft reinforcement.
Colorectal dysfunction frequently coexists in women with vault prolapse. Thus, a woman with extensive rectal prolapse should probably undergo concomitant Ripstein rectopexy and sacrocolpopexy, or a perineal proctosigmoidectomy and vaginal-approach vault suspension.
Careful and consistent preparation
Surgical success depends in great part on developing a clear understanding of anatomic defects and urodynamic dysfunction during the preoperative evaluation, to determine the most appropriate procedures.
Tissue preparation with low-dose estrogen
cream (1 g, two nights per week) is crucial for most postmenopausal women.
Obtain medical clearance, and optimize
perioperative safety by using spinal anesthesia, antiembolism stockings, and prophylactic intravenous antibiotics.
Retain vaginal packing at least 24 hours to prevent stress on sutures due to coughing or vomiting.
Advise patients in advance that, for 6 weeks after surgery, they must avoid overexertion and lifting more than 5 lb.
After 6 weeks, we restart estrogen cream and prescribe routine, daily Kegel exercise.
Vaginal procedures
McCall/Mayo culdoplasty
This involves plicating the uterosacral ligaments in the midline while reefing the peritoneum in the cul-de-sac, resulting in posterior culdoplasty. It usually is performed at the time of vaginal hysterectomy using nonabsorbable sutures to incorporate both uterosacral ligaments, intervening cul-desac peritoneum, and full-thickness apical vaginal mucosa. Multiple sutures may be required if prolapse is extensive.
Generally, we try to place our uppermost suture on the uterosacral ligaments at a distance from the cuff equal to the amount of vault prolapse (POP-Q: TVL minus point D [point C if uterus is absent]).
Be careful not to injure or kink the ureters when placing the suture through the uterosacral ligaments, as the ureters lie 1 to 2 cm lateral at the level of the cervix. We recommend cystoscopy with visualization of ureteral patency.
Success rates are high, but objective long-term data is scant.4,5
Uterosacral ligament suspension
Excellent anatomic outcomes have been described when the uterosacral ligaments are reattached to the vaginal apex (similar to the McCall technique).6,7 The physiologic nature of this technique makes it very attractive. It involves opening the vaginal wall from anterior to posterior over the apical defect, and identifying the pubocervical fascia, rectovaginal fascia, and uterosacral ligaments.
Technique. Place 1 permanent 1-0 suture and 1 delayed absorbable 1-0 suture in the posteromedial aspect of each uterosacral ligament 1 to 2 cm proximal and medial to each ischial spine. Then place 1 arm of each permanent suture through the pubocervical and rectovaginal fascia, and 1 arm of each delayed absorbable suture through the same tissue, also incorporating the vaginal epithelium. After repairing all additional defects, tie the sutures to suspend the vault.
When prolapse is extensive, redundant peritoneum can hinder identification of the uterosacral ligaments.
Success rates are 87% to 90%, but ureteral injury is a limiting factor, with rates as high as 11%. Therefore, cystoscopy is essential. Long-term data are lacking.
Iliococcygeus suspension
This safe and simple procedure involves elevating the vaginal apex to the iliococcygeus muscles along the lateral pelvic sidewall. This can be done without a vaginal incision by placing a monofilament permanent suture (polypropylene) full thickness through the vaginal wall into the muscle uni-or bilaterally.
Candidates should not be sexually active, as there will be a suture knot in the vagina. The procedure may be useful in elderly patients for whom complete restoration of vaginal anatomy is not a goal. It also can be performed as a salvage operation in women with suboptimal vault support and good distal vaginal anatomy. In addition, it can be performed following posterior vaginal wall dissection with entry into the pararectal space.
Technique. Place the sutures into the fascia overlying the iliococcygeus muscle, anterior to the ischial spine and inferior to the arcus tendineus fascia pelvis, and incorporate the pubocervical fascia anteriorly and the rectovaginal fascia posteriorly.
Success rates. Shull reported a 95% cure rate of the apical compartment among 42 women, at 6 weeks to 5 years.8 However, the prolapse at other sites was 14%. A randomized trial comparing this procedure to sacrospinous fixation demonstrated similar satisfactory outcomes.9
Sacrospinous ligament fixation
Probably the most commonly performed apical suspension procedure from the vaginal approach is fixation of the apex to the sacrospinous ligaments. Although many describe unilateral fixation, we advocate bilateral fixation to avoid lateral deviation of the vaginal axis (FIGURE 5).
Technique. After entering the pararectal space through a posterior vaginal wall dissection, identify the sacrospinous ligaments and place 2 nonabsorbable sutures through each ligament, rather than around it, as the pudendal vessels pass behind it.
Place the first suture 2 cm medial to the ischial spine, and the second suture 1 cm medial to the first. Then pass each suture through the underside of the vaginal apex—in the midline if the procedure is done unilaterally and under each apex if it is bilateral. When tied, the sutures suspend the vaginal apex by approximating it to the ligament, ideally without a suture bridge.
We use CV-2 GoreTex (WL Gore and Associates, Flagstaff, Ariz) sutures passed through the ligaments with a Miya hook, and we reinforce the underside of the vaginal apex with a rectangular piece of Prolene mesh (Ethicon, Somerville, NJ) if the mucosa is thinned.
Success rates are 70% to 97%.10,11 A significant concern is the nonanatomic posterior axial deflection of the vagina. Many investigators have reported an anterior compartment prolapse rate of up to 20% after fixation, likely secondary to increased force on the anterior compartment with increases in abdominal pressure. This is especially likely if a concomitant anti-incontinence procedure is performed.
Other complications include hemorrhage, vaginal shortening, sexual dysfunction, and buttock pain.
FIGURE 5 Bilateral sacrospinous fixation avoids lateral vaginal deviation
With bilateral fixation of the vault to the sacrospinous ligaments, the vaginal axis is more horizontal. It may be reinforced to enhance longevity. Reprinted with permission of The Cleveland Clinic Foundation.
Posterior IVS vault suspension
This novel, minimally invasive technique uses the posterior intravaginal slingplasty (Posterior IVS; Tyco/US Surgical, Norwalk, Conn). First described as infracoccygeal sacropexy, it was introduced as an outpatient procedure in Australia. Concerns about postoperative vaginal length and risk of rectal injury led to poor acceptance. The procedure was modified by a few US surgeons to enhance safety and vaginal length.
Technique. Enter the pararectal space in a fashion similar to that of sacrospinous fixation. A specially designed tunneler device delivers a multifilament polypropylene tape through bilateral perianal incisions. Secure the tape to the vaginal apex, and adjust it to provide vault support.
We modified this procedure to create neoligaments analogous to cardinal ligaments, by directing the tunneler through the iliococcygeus muscles in close proximity to the ischial spines and arcus tendineus. The resultant vaginal axis is physiologic, and vaginal length is normalized.
By combining this technique with perineoplasty and attaching the rectovaginal and pubocervical fascia to the tape, all levels of pelvic support are repaired once the vault is positioned by pulling on the perianal tape ends.
The new Apogee technique (American Medical Systems, Minnetonka, Minn) uses a similar perianal approach with monofilament polypropylene mesh.
Success rates. Preliminary success rates are 88% to 100%, and complication rates are minimal.12 Vaginal length averages 7 to 8 cm. Most initially reported complications involved graft erosion or rejection; shifting from nylon to polypropylene graft material reduced this problem.
Abdominal procedures
Sacral colpopexy
Considered the gold standard, the sacral colpopexy vaginal vault suspension technique has a consistent cure rate above 90%.13 It may be the ideal procedure for pelvic floor muscle weakness and/or attenuated fascia with multiple defects, for women for whom optimal sexual function is critical, and for those with other indications for abdominal surgery.
A graft is placed between the vagina and the sacral promontory to restore vaginal support (FIGURE 6). Materials have included autologous and synthetic materials. We use polypropylene mesh because of its high tensile strength, biocompatibility, low infection rate, and low incidence of erosion. Biologic grafts such as cadaveric fascia lata have increased failure rates due to graft breakdown.
The resultant vaginal axis is the most physiologic of all vault reconstructive procedures. This procedure appears to have the best longevity of all vault suspension procedures. It can be performed laparo-scopically at selected centers.
Technique. First, access the presacral space overlying the sacral promontory, taking care not to disturb the presacral and middle sacral vessels. We perform this step first to avoid potential periosteal tissue contamination. We routinely use 2 bone anchors to secure the mesh—making sterility imperative. Bone anchors reduce periosteal tissue trauma and decrease risk of potentially life-threatening hemorrhage.
Mobilize the bladder from the anterior vaginal apex. Repair any apical fascial defects, restoring continuity of the pubocervical and rectovaginal fascia, which often detach from the apex. Using 2-0 Prolene sutures, suture the y-shaped graft to both the anterior and posterior vaginal walls, incorporating all fascial edges.
Culdoplasty follows; this obliterates the cul-de-sac to prevent subsequent enterocele formation.
Next, place the graft in a tension-free manner, creating a suspensory bridge from the apex to the sacral promontory. Irrigate copiously. Close the peritoneum over the graft along its entire length.
Follow with any anti-incontinence and paravaginal support procedures as well as posterior colporrhaphy as needed.14,15
Major complications include hemorrhage, usually involving periosteal perforators along the sacrum. Graft erosion may affect up to 5% to 7% of sacral colpopexies.
FIGURE 6 Mesh bridge aids vault suspension
Abdominal sacrocolpopexy with a mesh bridge from the vaginal apex to the sacral promontory. Reprinted with permission of The Cleveland Clinic Foundation.
Uterosacral ligament suspension
In this procedure, which can be performed open or laparoscopically, the remnants of the uterosacral ligaments suspend the vaginal apex. The laparoscopic procedure is simple, especially if the uterus is in place.
Technique. Identify the course of the ureters in relation to the ligaments, and use nonabsorbable sutures to incorporate both of the uterosacral ligaments, peritoneum, and the vaginal apex—including the pubocervical and rectovaginal fascia (FIGURE 7).
Place multiple sutures (include the posterior vaginal wall) to obliterate the cul-de-sac and prevent enterocele development.
Success rates. Long-term data are minimal, but outcomes should be similar to the vaginal-approach culdoplasty.
FIGURE 7 Suspension from uterosacral ligaments
Laparoscopic uterosacral ligament suspension incorporating both uterosacral ligaments and cervix or vaginal cuff.
Reprinted with permission of The Cleveland Clinic Foundation.
Obliterative procedures
LeForte colpocleisis or colpectomy/vaginectomy are the simplest treatments for advanced prolapse in elderly women who are not—and will not be—sexually active.16
We prefer the LeForte colpocleisis, in which rectangular segments of the anterior and posterior vaginal walls are denuded of their epithelium, followed by approximation of the rectangles to one another.
Success rates exceed 95%, and safety is maintained if spinal anesthesia is used in conjunction with a high perineoplasty.
Dr. Biller reports no relevant financial relationships. Dr. Davila reports research support from AMS and Tyco/US Surgical. He also serves as a consultant to AMS, and as a speaker for AMS and Tyco/US Surgical.
1. Olsen AL, Smith VJ, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. Nygaard I, Bradley C, Brandt D, et al. Pelvic organ prolapse in older women: Prevalence and risk factors. Obstet Gynecol. 2004;104:489-497.
3. Thakar R, Stanton S. Management of genital prolapse. BMJ. 2002;324:1258-1262.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy. A preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Webb MJ, Aronson MP, et al. Posthysterectomy vaginal vault prolapse: primary repair in 693 patients. Obstet Gynecol. 1998;92:281-285.
6. Shull BL, Bachofen C, et al. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
7. Barber MD, Visco AG, et al. Bilateral uterosacral ligament vaginal vault suspension with site specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
8. Shull BL, Capen CV, et al. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 1993;168:1669-1677.
9. Maher CF, Murray CJ, et al. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98:40-44.
10. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872.-
11. Shull BL, Capen CV, et al. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
12. Davila GW, Miller D. Vaginal vault suspension using the Posterior IVS technique. J Pelvic Med Surg. 2004;10:S39.-
13. Addison WA, Bump RC, et al. Sacral colpopexy is the preferred treatment for vaginal vault prolapse in selected patients. J Gynecol Tech. 1996;2:69-74.
14. Kohli N, Walsh PM, et al. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92:999-1004.
15. Visco AG, Weidner AC, et al. Vaginal mesh erosion after abdominal sacral colpopexy. Am J Obstet Gynecol. 2001;184:297-302.
16. Neimark M, Davila GW, Kopka SL. LeForte colpocleisis: a feasible treatment option for pelvic organ prolapse in the elderly woman. J Pelvic Med Surg. 2003;9:1-7.
- Look for vault prolapse in any woman who has an advanced degree of vaginal prolapse.
- Goals of surgery: to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function.
- If sexual function is critical to the patient, a sacrocolpopexy should be the primary surgical option.
- Preoperative low-dose estrogen cream is crucial in most postmenopausal women.
Identifying vault prolapse can be difficult in a woman with extensive vaginal prolapse, and operative failure is likely if support to the apex is not restored.
Because this condition is so challenging to identify, many women undergoing anterior and/or posterior colporrhaphy likely have undiagnosed vault prolapse. This may contribute to the 29.2% rate of reoperation in women who undergo pelvic floor reconstructive procedures.1
This article reviews the anatomy of apical support, tells how to identify vaginal vault prolapse during the physical exam, and outlines effective surgical options—both vaginal and abdominal—for its correction. We focus on accurate pelvic assessment as the basis for planning the surgery.
Vaginal stability is fragile
The stability of vaginal anatomy is precarious, since it depends on a series of interrelationships between both dynamic and static structures. When the relationships between the ligaments and fascia at the vaginal apex or vault are impaired, vault prolapse ensues.
Thanks to cadaveric and radiographic studies, our understanding of the complexities of vaginal anatomy has improved considerably; still, the area of vaginal support we least understand is the coalescence of ligaments and fascia at the vaginal apex or vault.
Grade II prolapse, at least, in 64.8%
An analysis of Women’s Health Initiative enrollees with an intact uterus found that 64.8% had at least grade II prolapse (ie, leading edge of prolapse at –1 to +1 cm from the hymen) according to the Pelvic Organ Prolapse Quantification System (POP-Q).2 Approximately 8% of enrollees had a point D (vaginal apex) of greater than –6 cm, suggesting some degree of vault prolapse.
Hysterectomy appears to contribute. The incidence is about 1% at 3 years; 5% at 17 years.3
In the United States, approximately 30,000 vaginal vault repairs were performed in 1999.
Normal support structure
Several support structures coalesce at the vaginal apex. If the cervix is present, it serves as an obvious strong attachment site (FIGURE 1). In hysterectomized women, the structures may lack a strong attachment site, resulting in weakness and prolapse.
FIGURE 1 Vaginal support system
The coalescence of both sets of ligaments forms the uterosacral-cardinal ligament complex at the vaginal apex, which is likely crucial to vault support. Reprinted with permission of The Cleveland Clinic Foundation.
2 sets of ligaments determine support
Uterosacral ligaments—peritoneal and fibromuscular tissue bands extending from the vaginal apex to the sacrum—are the principal support for the vaginal apex, despite their apparent lack of strength.
The role of the cardinal ligaments—which extend laterally from the apex to the pelvic sidewall, adjacent to the ischial spine—is less clear. Since they lie proximal to the ureters, restoring vault support by shortening or reattaching them to the apex is a less attractive option.
The coalescence of these 2 sets of ligaments forms the complex that likely maintains vault support.
In hysterectomized women, locating the attachment of this complex to the vaginal cuff (seen on the exam as apical “dimples”) is key to identifying vault prolapse.
New view of cystoceles, rectoceles
The fibromuscular tissue layer underlying the vaginal epithelium envelops the entire vaginal canal, extending from apex to perineum and from arcus tendineus to arcus tendineus.
As the aponeurosis does for the abdominal wall, the endopelvic fascia maintains integrity of the anterior and posterior vaginal walls. If the fascial layer detaches from the vaginal apex, a true hernia can develop in the form of an enterocele—anterior or posterior—further weakening vault integrity (FIGURE 2).
Reconstructive surgeons are beginning to view cystoceles and rectoceles as a detachment of the endopelvic fascia from the vaginal apex. Thus, it is critical to restore anterior and posterior vaginal wall fascial integrity from apex to perineum by reattaching the endogenous fascia to the vaginal apex, or by placing a biologic or synthetic graft.
FIGURE 2 Apical defects contribute to vault prolapse
Vault prolapse is often associated with defects of the apical fascia, represented here by dark lines, which must be addressed during vault reconstruction. Reprinted with permission of The Cleveland Clinic Foundation.
Specific technique, tools to help identify prolapse
Any patient with an advanced degree of vaginal prolapse should be assessed for vault prolapse using a careful, structured pelvic exam. In many cases, this can be difficult, even if the uterus is present.
Necessary tools include a bivalved speculum and a right-angle retractor, or the posterior blade of another gynecologic speculum.
When the uterus is present
An exteriorized cervix does not necessarily mean vault prolapse; this may occur with substantial cervical hypertrophy, while the apex remains well supported (FIGURE 3).
Exam technique. Place the right-angle speculum blade in the posterior fornix, inserting it to its full extent, and ask the patient to perform a Valsalva maneuver. If vault prolapse is present, the uterus will descend further as the speculum is slowly removed; reinsertion of the speculum will resuspend the uterus. If the vault is well supported, the cervix will remain in place despite Valsalva efforts.
Assess the degree of vault prolapse during this examination, to determine whether a McCall culdoplasty will restore vault support.
If uterine suspension is performed in a woman with substantial cervical hypertrophy, cervical prolapse may persist, necessitating partial amputation (Manchester procedure).
FIGURE 3 Exteriorized cervix does not necessarily mean vault prolapse
Cervical prolapse may be associated with vault prolapse (left) or simply represent cervical hypertrophy without vault prolapse (right). Reprinted with permission of The Cleveland Clinic Foundation.
In the hysterectomized patient
The goal of physical exam is to identify the apical scar tissue (cuff) resultant from the hysterectomy. In most women, the cuff is visible as a transverse band of tissue firmer than the adjacent vaginal walls. If the woman has extensive prolapse, the tissue is stretched and thus not as obvious.
Exam technique. Use a bivalved speculum to visualize the apex. In women with extensive prolapse, redundant vaginal tissue may impede visualization. Fortunately, the sites of previous attachment of the uterosacral-cardinal ligament complex can usually be identified as “dimples” on either side of the midline at the cuff (FIGURE 4).
Use both right-angle speculum blades, or 1 blade along the anterior vaginal wall and the index and middle fingers of your other hand along the posterior vaginal wall, to identify the dimples. Then place the tip of the speculum between the dimples, elevate the vault while the patient performs a Valsalva effort, and determine the degree of vault prolapse. This can be confirmed by digital exam by identifying the dimples by tact and elevating them to their ipsilateral ischial spines.
FIGURE 4 Identifying the vault in the hysterectomized patient
Posthysterectomy vault prolapse can be identified by looking for “dimples” at the apex, which represent sites of previous uterosacral-cardinal ligament complex attachment. Reprinted with permission of The Cleveland Clinic Foundation.
Which exam findings point to which technique?
The importance of accurate pelvic assessment is impossible to overemphasize. Besides determining the degree and type of prolapse present, the exam enhances surgical planning. Fascial tears or defects are usually identifiable during careful vaginal exam as areas of sudden change in the thickness of the vaginal wall.
By the end of the pelvic exam, we usually have developed a surgical plan for the prolapse repair, pending urodynamic assessment to determine the best anti-incontinence procedure, if necessary.
What are the surgical goals?
Objectives are to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function—without precipitating new support or functional problems.
Abdominal versus vaginal approach
Most surgeons prefer a vaginal approach to pelvic reconstruction. However, this decision should be based on the patient’s individual variables.
If sexual function is critical to the patient, a sacrocolpopexy should be the primary option. Note that age does not always predict the importance of sexual function.
Vaginal length. If the vaginal apex (dimples) reaches the ischial spines with ease, a vaginal procedure should suffice. If it does not reach the spines, or extends far above, an abdominal sacrocolpopexy or obliterative procedure may more be appropriate.
Previous reconstructive procedures. Keep in mind that the area around the sacral promontory, or sacrospinous ligaments, may be difficult or risky to reach due to scarring and fibrosis. This is doubly important in this age of commonplace graft use.
Large paravaginal defects. Vaginal repairs can be technically difficult, and long-term outcomes have not been reported. An abdominal approach is probably better if substantial paravaginal defects are present.
Medical comorbidities. Use a vaginal or obliterative procedure under regional anesthesia if the patient is medically delicate or elderly.
Tissue quality usually improves with preoperative local estrogen, but large fascial defects adjacent to the cuff or perineum may require graft reinforcement.
Colorectal dysfunction frequently coexists in women with vault prolapse. Thus, a woman with extensive rectal prolapse should probably undergo concomitant Ripstein rectopexy and sacrocolpopexy, or a perineal proctosigmoidectomy and vaginal-approach vault suspension.
Careful and consistent preparation
Surgical success depends in great part on developing a clear understanding of anatomic defects and urodynamic dysfunction during the preoperative evaluation, to determine the most appropriate procedures.
Tissue preparation with low-dose estrogen
cream (1 g, two nights per week) is crucial for most postmenopausal women.
Obtain medical clearance, and optimize
perioperative safety by using spinal anesthesia, antiembolism stockings, and prophylactic intravenous antibiotics.
Retain vaginal packing at least 24 hours to prevent stress on sutures due to coughing or vomiting.
Advise patients in advance that, for 6 weeks after surgery, they must avoid overexertion and lifting more than 5 lb.
After 6 weeks, we restart estrogen cream and prescribe routine, daily Kegel exercise.
Vaginal procedures
McCall/Mayo culdoplasty
This involves plicating the uterosacral ligaments in the midline while reefing the peritoneum in the cul-de-sac, resulting in posterior culdoplasty. It usually is performed at the time of vaginal hysterectomy using nonabsorbable sutures to incorporate both uterosacral ligaments, intervening cul-desac peritoneum, and full-thickness apical vaginal mucosa. Multiple sutures may be required if prolapse is extensive.
Generally, we try to place our uppermost suture on the uterosacral ligaments at a distance from the cuff equal to the amount of vault prolapse (POP-Q: TVL minus point D [point C if uterus is absent]).
Be careful not to injure or kink the ureters when placing the suture through the uterosacral ligaments, as the ureters lie 1 to 2 cm lateral at the level of the cervix. We recommend cystoscopy with visualization of ureteral patency.
Success rates are high, but objective long-term data is scant.4,5
Uterosacral ligament suspension
Excellent anatomic outcomes have been described when the uterosacral ligaments are reattached to the vaginal apex (similar to the McCall technique).6,7 The physiologic nature of this technique makes it very attractive. It involves opening the vaginal wall from anterior to posterior over the apical defect, and identifying the pubocervical fascia, rectovaginal fascia, and uterosacral ligaments.
Technique. Place 1 permanent 1-0 suture and 1 delayed absorbable 1-0 suture in the posteromedial aspect of each uterosacral ligament 1 to 2 cm proximal and medial to each ischial spine. Then place 1 arm of each permanent suture through the pubocervical and rectovaginal fascia, and 1 arm of each delayed absorbable suture through the same tissue, also incorporating the vaginal epithelium. After repairing all additional defects, tie the sutures to suspend the vault.
When prolapse is extensive, redundant peritoneum can hinder identification of the uterosacral ligaments.
Success rates are 87% to 90%, but ureteral injury is a limiting factor, with rates as high as 11%. Therefore, cystoscopy is essential. Long-term data are lacking.
Iliococcygeus suspension
This safe and simple procedure involves elevating the vaginal apex to the iliococcygeus muscles along the lateral pelvic sidewall. This can be done without a vaginal incision by placing a monofilament permanent suture (polypropylene) full thickness through the vaginal wall into the muscle uni-or bilaterally.
Candidates should not be sexually active, as there will be a suture knot in the vagina. The procedure may be useful in elderly patients for whom complete restoration of vaginal anatomy is not a goal. It also can be performed as a salvage operation in women with suboptimal vault support and good distal vaginal anatomy. In addition, it can be performed following posterior vaginal wall dissection with entry into the pararectal space.
Technique. Place the sutures into the fascia overlying the iliococcygeus muscle, anterior to the ischial spine and inferior to the arcus tendineus fascia pelvis, and incorporate the pubocervical fascia anteriorly and the rectovaginal fascia posteriorly.
Success rates. Shull reported a 95% cure rate of the apical compartment among 42 women, at 6 weeks to 5 years.8 However, the prolapse at other sites was 14%. A randomized trial comparing this procedure to sacrospinous fixation demonstrated similar satisfactory outcomes.9
Sacrospinous ligament fixation
Probably the most commonly performed apical suspension procedure from the vaginal approach is fixation of the apex to the sacrospinous ligaments. Although many describe unilateral fixation, we advocate bilateral fixation to avoid lateral deviation of the vaginal axis (FIGURE 5).
Technique. After entering the pararectal space through a posterior vaginal wall dissection, identify the sacrospinous ligaments and place 2 nonabsorbable sutures through each ligament, rather than around it, as the pudendal vessels pass behind it.
Place the first suture 2 cm medial to the ischial spine, and the second suture 1 cm medial to the first. Then pass each suture through the underside of the vaginal apex—in the midline if the procedure is done unilaterally and under each apex if it is bilateral. When tied, the sutures suspend the vaginal apex by approximating it to the ligament, ideally without a suture bridge.
We use CV-2 GoreTex (WL Gore and Associates, Flagstaff, Ariz) sutures passed through the ligaments with a Miya hook, and we reinforce the underside of the vaginal apex with a rectangular piece of Prolene mesh (Ethicon, Somerville, NJ) if the mucosa is thinned.
Success rates are 70% to 97%.10,11 A significant concern is the nonanatomic posterior axial deflection of the vagina. Many investigators have reported an anterior compartment prolapse rate of up to 20% after fixation, likely secondary to increased force on the anterior compartment with increases in abdominal pressure. This is especially likely if a concomitant anti-incontinence procedure is performed.
Other complications include hemorrhage, vaginal shortening, sexual dysfunction, and buttock pain.
FIGURE 5 Bilateral sacrospinous fixation avoids lateral vaginal deviation
With bilateral fixation of the vault to the sacrospinous ligaments, the vaginal axis is more horizontal. It may be reinforced to enhance longevity. Reprinted with permission of The Cleveland Clinic Foundation.
Posterior IVS vault suspension
This novel, minimally invasive technique uses the posterior intravaginal slingplasty (Posterior IVS; Tyco/US Surgical, Norwalk, Conn). First described as infracoccygeal sacropexy, it was introduced as an outpatient procedure in Australia. Concerns about postoperative vaginal length and risk of rectal injury led to poor acceptance. The procedure was modified by a few US surgeons to enhance safety and vaginal length.
Technique. Enter the pararectal space in a fashion similar to that of sacrospinous fixation. A specially designed tunneler device delivers a multifilament polypropylene tape through bilateral perianal incisions. Secure the tape to the vaginal apex, and adjust it to provide vault support.
We modified this procedure to create neoligaments analogous to cardinal ligaments, by directing the tunneler through the iliococcygeus muscles in close proximity to the ischial spines and arcus tendineus. The resultant vaginal axis is physiologic, and vaginal length is normalized.
By combining this technique with perineoplasty and attaching the rectovaginal and pubocervical fascia to the tape, all levels of pelvic support are repaired once the vault is positioned by pulling on the perianal tape ends.
The new Apogee technique (American Medical Systems, Minnetonka, Minn) uses a similar perianal approach with monofilament polypropylene mesh.
Success rates. Preliminary success rates are 88% to 100%, and complication rates are minimal.12 Vaginal length averages 7 to 8 cm. Most initially reported complications involved graft erosion or rejection; shifting from nylon to polypropylene graft material reduced this problem.
Abdominal procedures
Sacral colpopexy
Considered the gold standard, the sacral colpopexy vaginal vault suspension technique has a consistent cure rate above 90%.13 It may be the ideal procedure for pelvic floor muscle weakness and/or attenuated fascia with multiple defects, for women for whom optimal sexual function is critical, and for those with other indications for abdominal surgery.
A graft is placed between the vagina and the sacral promontory to restore vaginal support (FIGURE 6). Materials have included autologous and synthetic materials. We use polypropylene mesh because of its high tensile strength, biocompatibility, low infection rate, and low incidence of erosion. Biologic grafts such as cadaveric fascia lata have increased failure rates due to graft breakdown.
The resultant vaginal axis is the most physiologic of all vault reconstructive procedures. This procedure appears to have the best longevity of all vault suspension procedures. It can be performed laparo-scopically at selected centers.
Technique. First, access the presacral space overlying the sacral promontory, taking care not to disturb the presacral and middle sacral vessels. We perform this step first to avoid potential periosteal tissue contamination. We routinely use 2 bone anchors to secure the mesh—making sterility imperative. Bone anchors reduce periosteal tissue trauma and decrease risk of potentially life-threatening hemorrhage.
Mobilize the bladder from the anterior vaginal apex. Repair any apical fascial defects, restoring continuity of the pubocervical and rectovaginal fascia, which often detach from the apex. Using 2-0 Prolene sutures, suture the y-shaped graft to both the anterior and posterior vaginal walls, incorporating all fascial edges.
Culdoplasty follows; this obliterates the cul-de-sac to prevent subsequent enterocele formation.
Next, place the graft in a tension-free manner, creating a suspensory bridge from the apex to the sacral promontory. Irrigate copiously. Close the peritoneum over the graft along its entire length.
Follow with any anti-incontinence and paravaginal support procedures as well as posterior colporrhaphy as needed.14,15
Major complications include hemorrhage, usually involving periosteal perforators along the sacrum. Graft erosion may affect up to 5% to 7% of sacral colpopexies.
FIGURE 6 Mesh bridge aids vault suspension
Abdominal sacrocolpopexy with a mesh bridge from the vaginal apex to the sacral promontory. Reprinted with permission of The Cleveland Clinic Foundation.
Uterosacral ligament suspension
In this procedure, which can be performed open or laparoscopically, the remnants of the uterosacral ligaments suspend the vaginal apex. The laparoscopic procedure is simple, especially if the uterus is in place.
Technique. Identify the course of the ureters in relation to the ligaments, and use nonabsorbable sutures to incorporate both of the uterosacral ligaments, peritoneum, and the vaginal apex—including the pubocervical and rectovaginal fascia (FIGURE 7).
Place multiple sutures (include the posterior vaginal wall) to obliterate the cul-de-sac and prevent enterocele development.
Success rates. Long-term data are minimal, but outcomes should be similar to the vaginal-approach culdoplasty.
FIGURE 7 Suspension from uterosacral ligaments
Laparoscopic uterosacral ligament suspension incorporating both uterosacral ligaments and cervix or vaginal cuff.
Reprinted with permission of The Cleveland Clinic Foundation.
Obliterative procedures
LeForte colpocleisis or colpectomy/vaginectomy are the simplest treatments for advanced prolapse in elderly women who are not—and will not be—sexually active.16
We prefer the LeForte colpocleisis, in which rectangular segments of the anterior and posterior vaginal walls are denuded of their epithelium, followed by approximation of the rectangles to one another.
Success rates exceed 95%, and safety is maintained if spinal anesthesia is used in conjunction with a high perineoplasty.
Dr. Biller reports no relevant financial relationships. Dr. Davila reports research support from AMS and Tyco/US Surgical. He also serves as a consultant to AMS, and as a speaker for AMS and Tyco/US Surgical.
- Look for vault prolapse in any woman who has an advanced degree of vaginal prolapse.
- Goals of surgery: to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function.
- If sexual function is critical to the patient, a sacrocolpopexy should be the primary surgical option.
- Preoperative low-dose estrogen cream is crucial in most postmenopausal women.
Identifying vault prolapse can be difficult in a woman with extensive vaginal prolapse, and operative failure is likely if support to the apex is not restored.
Because this condition is so challenging to identify, many women undergoing anterior and/or posterior colporrhaphy likely have undiagnosed vault prolapse. This may contribute to the 29.2% rate of reoperation in women who undergo pelvic floor reconstructive procedures.1
This article reviews the anatomy of apical support, tells how to identify vaginal vault prolapse during the physical exam, and outlines effective surgical options—both vaginal and abdominal—for its correction. We focus on accurate pelvic assessment as the basis for planning the surgery.
Vaginal stability is fragile
The stability of vaginal anatomy is precarious, since it depends on a series of interrelationships between both dynamic and static structures. When the relationships between the ligaments and fascia at the vaginal apex or vault are impaired, vault prolapse ensues.
Thanks to cadaveric and radiographic studies, our understanding of the complexities of vaginal anatomy has improved considerably; still, the area of vaginal support we least understand is the coalescence of ligaments and fascia at the vaginal apex or vault.
Grade II prolapse, at least, in 64.8%
An analysis of Women’s Health Initiative enrollees with an intact uterus found that 64.8% had at least grade II prolapse (ie, leading edge of prolapse at –1 to +1 cm from the hymen) according to the Pelvic Organ Prolapse Quantification System (POP-Q).2 Approximately 8% of enrollees had a point D (vaginal apex) of greater than –6 cm, suggesting some degree of vault prolapse.
Hysterectomy appears to contribute. The incidence is about 1% at 3 years; 5% at 17 years.3
In the United States, approximately 30,000 vaginal vault repairs were performed in 1999.
Normal support structure
Several support structures coalesce at the vaginal apex. If the cervix is present, it serves as an obvious strong attachment site (FIGURE 1). In hysterectomized women, the structures may lack a strong attachment site, resulting in weakness and prolapse.
FIGURE 1 Vaginal support system
The coalescence of both sets of ligaments forms the uterosacral-cardinal ligament complex at the vaginal apex, which is likely crucial to vault support. Reprinted with permission of The Cleveland Clinic Foundation.
2 sets of ligaments determine support
Uterosacral ligaments—peritoneal and fibromuscular tissue bands extending from the vaginal apex to the sacrum—are the principal support for the vaginal apex, despite their apparent lack of strength.
The role of the cardinal ligaments—which extend laterally from the apex to the pelvic sidewall, adjacent to the ischial spine—is less clear. Since they lie proximal to the ureters, restoring vault support by shortening or reattaching them to the apex is a less attractive option.
The coalescence of these 2 sets of ligaments forms the complex that likely maintains vault support.
In hysterectomized women, locating the attachment of this complex to the vaginal cuff (seen on the exam as apical “dimples”) is key to identifying vault prolapse.
New view of cystoceles, rectoceles
The fibromuscular tissue layer underlying the vaginal epithelium envelops the entire vaginal canal, extending from apex to perineum and from arcus tendineus to arcus tendineus.
As the aponeurosis does for the abdominal wall, the endopelvic fascia maintains integrity of the anterior and posterior vaginal walls. If the fascial layer detaches from the vaginal apex, a true hernia can develop in the form of an enterocele—anterior or posterior—further weakening vault integrity (FIGURE 2).
Reconstructive surgeons are beginning to view cystoceles and rectoceles as a detachment of the endopelvic fascia from the vaginal apex. Thus, it is critical to restore anterior and posterior vaginal wall fascial integrity from apex to perineum by reattaching the endogenous fascia to the vaginal apex, or by placing a biologic or synthetic graft.
FIGURE 2 Apical defects contribute to vault prolapse
Vault prolapse is often associated with defects of the apical fascia, represented here by dark lines, which must be addressed during vault reconstruction. Reprinted with permission of The Cleveland Clinic Foundation.
Specific technique, tools to help identify prolapse
Any patient with an advanced degree of vaginal prolapse should be assessed for vault prolapse using a careful, structured pelvic exam. In many cases, this can be difficult, even if the uterus is present.
Necessary tools include a bivalved speculum and a right-angle retractor, or the posterior blade of another gynecologic speculum.
When the uterus is present
An exteriorized cervix does not necessarily mean vault prolapse; this may occur with substantial cervical hypertrophy, while the apex remains well supported (FIGURE 3).
Exam technique. Place the right-angle speculum blade in the posterior fornix, inserting it to its full extent, and ask the patient to perform a Valsalva maneuver. If vault prolapse is present, the uterus will descend further as the speculum is slowly removed; reinsertion of the speculum will resuspend the uterus. If the vault is well supported, the cervix will remain in place despite Valsalva efforts.
Assess the degree of vault prolapse during this examination, to determine whether a McCall culdoplasty will restore vault support.
If uterine suspension is performed in a woman with substantial cervical hypertrophy, cervical prolapse may persist, necessitating partial amputation (Manchester procedure).
FIGURE 3 Exteriorized cervix does not necessarily mean vault prolapse
Cervical prolapse may be associated with vault prolapse (left) or simply represent cervical hypertrophy without vault prolapse (right). Reprinted with permission of The Cleveland Clinic Foundation.
In the hysterectomized patient
The goal of physical exam is to identify the apical scar tissue (cuff) resultant from the hysterectomy. In most women, the cuff is visible as a transverse band of tissue firmer than the adjacent vaginal walls. If the woman has extensive prolapse, the tissue is stretched and thus not as obvious.
Exam technique. Use a bivalved speculum to visualize the apex. In women with extensive prolapse, redundant vaginal tissue may impede visualization. Fortunately, the sites of previous attachment of the uterosacral-cardinal ligament complex can usually be identified as “dimples” on either side of the midline at the cuff (FIGURE 4).
Use both right-angle speculum blades, or 1 blade along the anterior vaginal wall and the index and middle fingers of your other hand along the posterior vaginal wall, to identify the dimples. Then place the tip of the speculum between the dimples, elevate the vault while the patient performs a Valsalva effort, and determine the degree of vault prolapse. This can be confirmed by digital exam by identifying the dimples by tact and elevating them to their ipsilateral ischial spines.
FIGURE 4 Identifying the vault in the hysterectomized patient
Posthysterectomy vault prolapse can be identified by looking for “dimples” at the apex, which represent sites of previous uterosacral-cardinal ligament complex attachment. Reprinted with permission of The Cleveland Clinic Foundation.
Which exam findings point to which technique?
The importance of accurate pelvic assessment is impossible to overemphasize. Besides determining the degree and type of prolapse present, the exam enhances surgical planning. Fascial tears or defects are usually identifiable during careful vaginal exam as areas of sudden change in the thickness of the vaginal wall.
By the end of the pelvic exam, we usually have developed a surgical plan for the prolapse repair, pending urodynamic assessment to determine the best anti-incontinence procedure, if necessary.
What are the surgical goals?
Objectives are to normalize support of all anatomic compartments; alleviate clinical symptoms; and optimize sexual, bowel, and bladder function—without precipitating new support or functional problems.
Abdominal versus vaginal approach
Most surgeons prefer a vaginal approach to pelvic reconstruction. However, this decision should be based on the patient’s individual variables.
If sexual function is critical to the patient, a sacrocolpopexy should be the primary option. Note that age does not always predict the importance of sexual function.
Vaginal length. If the vaginal apex (dimples) reaches the ischial spines with ease, a vaginal procedure should suffice. If it does not reach the spines, or extends far above, an abdominal sacrocolpopexy or obliterative procedure may more be appropriate.
Previous reconstructive procedures. Keep in mind that the area around the sacral promontory, or sacrospinous ligaments, may be difficult or risky to reach due to scarring and fibrosis. This is doubly important in this age of commonplace graft use.
Large paravaginal defects. Vaginal repairs can be technically difficult, and long-term outcomes have not been reported. An abdominal approach is probably better if substantial paravaginal defects are present.
Medical comorbidities. Use a vaginal or obliterative procedure under regional anesthesia if the patient is medically delicate or elderly.
Tissue quality usually improves with preoperative local estrogen, but large fascial defects adjacent to the cuff or perineum may require graft reinforcement.
Colorectal dysfunction frequently coexists in women with vault prolapse. Thus, a woman with extensive rectal prolapse should probably undergo concomitant Ripstein rectopexy and sacrocolpopexy, or a perineal proctosigmoidectomy and vaginal-approach vault suspension.
Careful and consistent preparation
Surgical success depends in great part on developing a clear understanding of anatomic defects and urodynamic dysfunction during the preoperative evaluation, to determine the most appropriate procedures.
Tissue preparation with low-dose estrogen
cream (1 g, two nights per week) is crucial for most postmenopausal women.
Obtain medical clearance, and optimize
perioperative safety by using spinal anesthesia, antiembolism stockings, and prophylactic intravenous antibiotics.
Retain vaginal packing at least 24 hours to prevent stress on sutures due to coughing or vomiting.
Advise patients in advance that, for 6 weeks after surgery, they must avoid overexertion and lifting more than 5 lb.
After 6 weeks, we restart estrogen cream and prescribe routine, daily Kegel exercise.
Vaginal procedures
McCall/Mayo culdoplasty
This involves plicating the uterosacral ligaments in the midline while reefing the peritoneum in the cul-de-sac, resulting in posterior culdoplasty. It usually is performed at the time of vaginal hysterectomy using nonabsorbable sutures to incorporate both uterosacral ligaments, intervening cul-desac peritoneum, and full-thickness apical vaginal mucosa. Multiple sutures may be required if prolapse is extensive.
Generally, we try to place our uppermost suture on the uterosacral ligaments at a distance from the cuff equal to the amount of vault prolapse (POP-Q: TVL minus point D [point C if uterus is absent]).
Be careful not to injure or kink the ureters when placing the suture through the uterosacral ligaments, as the ureters lie 1 to 2 cm lateral at the level of the cervix. We recommend cystoscopy with visualization of ureteral patency.
Success rates are high, but objective long-term data is scant.4,5
Uterosacral ligament suspension
Excellent anatomic outcomes have been described when the uterosacral ligaments are reattached to the vaginal apex (similar to the McCall technique).6,7 The physiologic nature of this technique makes it very attractive. It involves opening the vaginal wall from anterior to posterior over the apical defect, and identifying the pubocervical fascia, rectovaginal fascia, and uterosacral ligaments.
Technique. Place 1 permanent 1-0 suture and 1 delayed absorbable 1-0 suture in the posteromedial aspect of each uterosacral ligament 1 to 2 cm proximal and medial to each ischial spine. Then place 1 arm of each permanent suture through the pubocervical and rectovaginal fascia, and 1 arm of each delayed absorbable suture through the same tissue, also incorporating the vaginal epithelium. After repairing all additional defects, tie the sutures to suspend the vault.
When prolapse is extensive, redundant peritoneum can hinder identification of the uterosacral ligaments.
Success rates are 87% to 90%, but ureteral injury is a limiting factor, with rates as high as 11%. Therefore, cystoscopy is essential. Long-term data are lacking.
Iliococcygeus suspension
This safe and simple procedure involves elevating the vaginal apex to the iliococcygeus muscles along the lateral pelvic sidewall. This can be done without a vaginal incision by placing a monofilament permanent suture (polypropylene) full thickness through the vaginal wall into the muscle uni-or bilaterally.
Candidates should not be sexually active, as there will be a suture knot in the vagina. The procedure may be useful in elderly patients for whom complete restoration of vaginal anatomy is not a goal. It also can be performed as a salvage operation in women with suboptimal vault support and good distal vaginal anatomy. In addition, it can be performed following posterior vaginal wall dissection with entry into the pararectal space.
Technique. Place the sutures into the fascia overlying the iliococcygeus muscle, anterior to the ischial spine and inferior to the arcus tendineus fascia pelvis, and incorporate the pubocervical fascia anteriorly and the rectovaginal fascia posteriorly.
Success rates. Shull reported a 95% cure rate of the apical compartment among 42 women, at 6 weeks to 5 years.8 However, the prolapse at other sites was 14%. A randomized trial comparing this procedure to sacrospinous fixation demonstrated similar satisfactory outcomes.9
Sacrospinous ligament fixation
Probably the most commonly performed apical suspension procedure from the vaginal approach is fixation of the apex to the sacrospinous ligaments. Although many describe unilateral fixation, we advocate bilateral fixation to avoid lateral deviation of the vaginal axis (FIGURE 5).
Technique. After entering the pararectal space through a posterior vaginal wall dissection, identify the sacrospinous ligaments and place 2 nonabsorbable sutures through each ligament, rather than around it, as the pudendal vessels pass behind it.
Place the first suture 2 cm medial to the ischial spine, and the second suture 1 cm medial to the first. Then pass each suture through the underside of the vaginal apex—in the midline if the procedure is done unilaterally and under each apex if it is bilateral. When tied, the sutures suspend the vaginal apex by approximating it to the ligament, ideally without a suture bridge.
We use CV-2 GoreTex (WL Gore and Associates, Flagstaff, Ariz) sutures passed through the ligaments with a Miya hook, and we reinforce the underside of the vaginal apex with a rectangular piece of Prolene mesh (Ethicon, Somerville, NJ) if the mucosa is thinned.
Success rates are 70% to 97%.10,11 A significant concern is the nonanatomic posterior axial deflection of the vagina. Many investigators have reported an anterior compartment prolapse rate of up to 20% after fixation, likely secondary to increased force on the anterior compartment with increases in abdominal pressure. This is especially likely if a concomitant anti-incontinence procedure is performed.
Other complications include hemorrhage, vaginal shortening, sexual dysfunction, and buttock pain.
FIGURE 5 Bilateral sacrospinous fixation avoids lateral vaginal deviation
With bilateral fixation of the vault to the sacrospinous ligaments, the vaginal axis is more horizontal. It may be reinforced to enhance longevity. Reprinted with permission of The Cleveland Clinic Foundation.
Posterior IVS vault suspension
This novel, minimally invasive technique uses the posterior intravaginal slingplasty (Posterior IVS; Tyco/US Surgical, Norwalk, Conn). First described as infracoccygeal sacropexy, it was introduced as an outpatient procedure in Australia. Concerns about postoperative vaginal length and risk of rectal injury led to poor acceptance. The procedure was modified by a few US surgeons to enhance safety and vaginal length.
Technique. Enter the pararectal space in a fashion similar to that of sacrospinous fixation. A specially designed tunneler device delivers a multifilament polypropylene tape through bilateral perianal incisions. Secure the tape to the vaginal apex, and adjust it to provide vault support.
We modified this procedure to create neoligaments analogous to cardinal ligaments, by directing the tunneler through the iliococcygeus muscles in close proximity to the ischial spines and arcus tendineus. The resultant vaginal axis is physiologic, and vaginal length is normalized.
By combining this technique with perineoplasty and attaching the rectovaginal and pubocervical fascia to the tape, all levels of pelvic support are repaired once the vault is positioned by pulling on the perianal tape ends.
The new Apogee technique (American Medical Systems, Minnetonka, Minn) uses a similar perianal approach with monofilament polypropylene mesh.
Success rates. Preliminary success rates are 88% to 100%, and complication rates are minimal.12 Vaginal length averages 7 to 8 cm. Most initially reported complications involved graft erosion or rejection; shifting from nylon to polypropylene graft material reduced this problem.
Abdominal procedures
Sacral colpopexy
Considered the gold standard, the sacral colpopexy vaginal vault suspension technique has a consistent cure rate above 90%.13 It may be the ideal procedure for pelvic floor muscle weakness and/or attenuated fascia with multiple defects, for women for whom optimal sexual function is critical, and for those with other indications for abdominal surgery.
A graft is placed between the vagina and the sacral promontory to restore vaginal support (FIGURE 6). Materials have included autologous and synthetic materials. We use polypropylene mesh because of its high tensile strength, biocompatibility, low infection rate, and low incidence of erosion. Biologic grafts such as cadaveric fascia lata have increased failure rates due to graft breakdown.
The resultant vaginal axis is the most physiologic of all vault reconstructive procedures. This procedure appears to have the best longevity of all vault suspension procedures. It can be performed laparo-scopically at selected centers.
Technique. First, access the presacral space overlying the sacral promontory, taking care not to disturb the presacral and middle sacral vessels. We perform this step first to avoid potential periosteal tissue contamination. We routinely use 2 bone anchors to secure the mesh—making sterility imperative. Bone anchors reduce periosteal tissue trauma and decrease risk of potentially life-threatening hemorrhage.
Mobilize the bladder from the anterior vaginal apex. Repair any apical fascial defects, restoring continuity of the pubocervical and rectovaginal fascia, which often detach from the apex. Using 2-0 Prolene sutures, suture the y-shaped graft to both the anterior and posterior vaginal walls, incorporating all fascial edges.
Culdoplasty follows; this obliterates the cul-de-sac to prevent subsequent enterocele formation.
Next, place the graft in a tension-free manner, creating a suspensory bridge from the apex to the sacral promontory. Irrigate copiously. Close the peritoneum over the graft along its entire length.
Follow with any anti-incontinence and paravaginal support procedures as well as posterior colporrhaphy as needed.14,15
Major complications include hemorrhage, usually involving periosteal perforators along the sacrum. Graft erosion may affect up to 5% to 7% of sacral colpopexies.
FIGURE 6 Mesh bridge aids vault suspension
Abdominal sacrocolpopexy with a mesh bridge from the vaginal apex to the sacral promontory. Reprinted with permission of The Cleveland Clinic Foundation.
Uterosacral ligament suspension
In this procedure, which can be performed open or laparoscopically, the remnants of the uterosacral ligaments suspend the vaginal apex. The laparoscopic procedure is simple, especially if the uterus is in place.
Technique. Identify the course of the ureters in relation to the ligaments, and use nonabsorbable sutures to incorporate both of the uterosacral ligaments, peritoneum, and the vaginal apex—including the pubocervical and rectovaginal fascia (FIGURE 7).
Place multiple sutures (include the posterior vaginal wall) to obliterate the cul-de-sac and prevent enterocele development.
Success rates. Long-term data are minimal, but outcomes should be similar to the vaginal-approach culdoplasty.
FIGURE 7 Suspension from uterosacral ligaments
Laparoscopic uterosacral ligament suspension incorporating both uterosacral ligaments and cervix or vaginal cuff.
Reprinted with permission of The Cleveland Clinic Foundation.
Obliterative procedures
LeForte colpocleisis or colpectomy/vaginectomy are the simplest treatments for advanced prolapse in elderly women who are not—and will not be—sexually active.16
We prefer the LeForte colpocleisis, in which rectangular segments of the anterior and posterior vaginal walls are denuded of their epithelium, followed by approximation of the rectangles to one another.
Success rates exceed 95%, and safety is maintained if spinal anesthesia is used in conjunction with a high perineoplasty.
Dr. Biller reports no relevant financial relationships. Dr. Davila reports research support from AMS and Tyco/US Surgical. He also serves as a consultant to AMS, and as a speaker for AMS and Tyco/US Surgical.
1. Olsen AL, Smith VJ, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. Nygaard I, Bradley C, Brandt D, et al. Pelvic organ prolapse in older women: Prevalence and risk factors. Obstet Gynecol. 2004;104:489-497.
3. Thakar R, Stanton S. Management of genital prolapse. BMJ. 2002;324:1258-1262.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy. A preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Webb MJ, Aronson MP, et al. Posthysterectomy vaginal vault prolapse: primary repair in 693 patients. Obstet Gynecol. 1998;92:281-285.
6. Shull BL, Bachofen C, et al. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
7. Barber MD, Visco AG, et al. Bilateral uterosacral ligament vaginal vault suspension with site specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
8. Shull BL, Capen CV, et al. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 1993;168:1669-1677.
9. Maher CF, Murray CJ, et al. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98:40-44.
10. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872.-
11. Shull BL, Capen CV, et al. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
12. Davila GW, Miller D. Vaginal vault suspension using the Posterior IVS technique. J Pelvic Med Surg. 2004;10:S39.-
13. Addison WA, Bump RC, et al. Sacral colpopexy is the preferred treatment for vaginal vault prolapse in selected patients. J Gynecol Tech. 1996;2:69-74.
14. Kohli N, Walsh PM, et al. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92:999-1004.
15. Visco AG, Weidner AC, et al. Vaginal mesh erosion after abdominal sacral colpopexy. Am J Obstet Gynecol. 2001;184:297-302.
16. Neimark M, Davila GW, Kopka SL. LeForte colpocleisis: a feasible treatment option for pelvic organ prolapse in the elderly woman. J Pelvic Med Surg. 2003;9:1-7.
1. Olsen AL, Smith VJ, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. Nygaard I, Bradley C, Brandt D, et al. Pelvic organ prolapse in older women: Prevalence and risk factors. Obstet Gynecol. 2004;104:489-497.
3. Thakar R, Stanton S. Management of genital prolapse. BMJ. 2002;324:1258-1262.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy. A preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Webb MJ, Aronson MP, et al. Posthysterectomy vaginal vault prolapse: primary repair in 693 patients. Obstet Gynecol. 1998;92:281-285.
6. Shull BL, Bachofen C, et al. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
7. Barber MD, Visco AG, et al. Bilateral uterosacral ligament vaginal vault suspension with site specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
8. Shull BL, Capen CV, et al. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 1993;168:1669-1677.
9. Maher CF, Murray CJ, et al. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98:40-44.
10. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872.-
11. Shull BL, Capen CV, et al. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
12. Davila GW, Miller D. Vaginal vault suspension using the Posterior IVS technique. J Pelvic Med Surg. 2004;10:S39.-
13. Addison WA, Bump RC, et al. Sacral colpopexy is the preferred treatment for vaginal vault prolapse in selected patients. J Gynecol Tech. 1996;2:69-74.
14. Kohli N, Walsh PM, et al. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92:999-1004.
15. Visco AG, Weidner AC, et al. Vaginal mesh erosion after abdominal sacral colpopexy. Am J Obstet Gynecol. 2001;184:297-302.
16. Neimark M, Davila GW, Kopka SL. LeForte colpocleisis: a feasible treatment option for pelvic organ prolapse in the elderly woman. J Pelvic Med Surg. 2003;9:1-7.