CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?
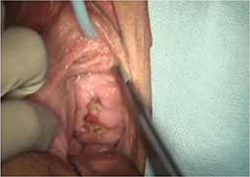
FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.


