User login
A Forgotten Cause of Cardiac Tamponade
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
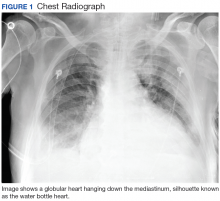
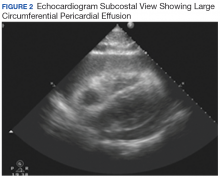
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
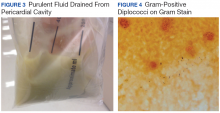
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
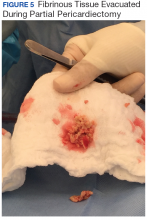
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
Misleading Diagnosis of Idiopathic Pulmonary Fibrosis: A Clinical Concern
Sjogren syndrome (SS) is a chronic inflammatory autoimmune disorder characterized by lymphocytic infiltration of lacrimal and salivary glands causing sicca syndrome.¹ The disease can extend beyond the exocrine glands, and systemic manifestations, including vasculitis, lung, renal or neurologic involvement, can occur.² Lung disease associated with SS is more commonly seen in women aged ≥ 60 years. The most common symptoms include dry cough, chest pain, and dyspnea on exertion. Sjogren syndrome also may produce several respiratory complications, including bronchial hyperresponsiveness, bronchiolitis, bronchiectasis, pulmonary infections, pulmonary amyloidosis, pulmonary embolism, pulmonary hypertension, lymphomas, and interstitial lung diseases (ILD).² Although ILD typically occurs 5 to 10 years after the onset of SS, lung disease can precede SS.
Pulmonary involvement is associated with systemic manifestations, hypergammaglobulinemia, and anti-SSA and anti-SSB antibodies.² Laboratory tests that confirm a diagnosis of SS include antinuclear antibody (ANA), anti-Ro/SSA, and anti-La/SSB antibodies.² Pulmonary function test (PFT) results appear to reflect impairment of either the lung (restrictive syndrome) or airways (obstructive syndrome).² Imaging abnormalities may include ground-glass attenuation, subpleural small nodules, nonseptal linear opacities, interlobular septal thickening, bronchiectasis, and cysts.³ Therefore, many ILD cases show similar imaging and pathologic findings; nevertheless, they have identifiable etiology that are not idiopathic.
Case Presentation
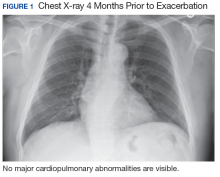
A 67-year-old man with a medical history of hypertension, peripheral vascular disease, and keratoconjunctivitis sicca (treated with eye drops) developed progressive shortness of breath, dyspnea on exertion, and weight loss (40 pounds) over the course of 6 months. A pulmonary function test showed a restrictive abnormality with decreased diffusing capacity of the lungs for carbon monoxide (eFigure available online at www.fedprac.com). A chest computed tomography (CT) scan showed the presence of significant thickening of the interlobular septi that was more pronounced in the subpleural regions of the lungs and lower lobes, which was consistent with usual interstitial pneumonia. A chest X-ray conducted 4 months prior showed no significant acute cardiopulmonary abnormalities (Figure 1). An open lung wedge biopsy revealed chronic organizing pneumonia with mild interstitial chronic inflammation, smooth muscle hypertrophy, and honeycomb changes consistent with usual interstitial pneumonia.
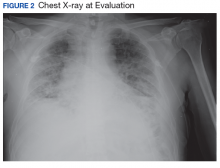
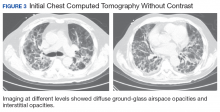
The patient had been diagnosed with idiopathic pulmonary fibrosis (IPF) by a private physician and started pirfenidone and oxygen therapy. Three months later the patient presented to the VA Caribbean Healthcare System in San Juan Puerto Rico when he developed an exacerbation of IPF. The patient reported having fever, chills, dry cough, night sweats, and marked shortness of breath. He was found hypoxemic (partial pressure of O2 was 50 mm Hg) and required a venturi mask set to 50% fractioned of inspired O2 to maintain a peripheral oxygen saturation around 90%. A chest X-ray showed decreased lung volume with bilateral interstitial and alveolar disease (Figure 2). Leukocytosis was present at 17×10-3/µl. The chest CT scan showed interval worsening of diffuse ground-glass airspace opacities and worsening of interstitial opacities; there
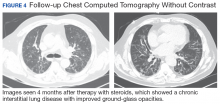
After careful clinical assessment (+ dry eyes) and radiographic pattern evaluation (diffuse bilateral interstitial and ground-glass opacities), the clinical diagnosis of IPF was queried after the patient’s rheumatologic workup came back positive for ANA and anti-Ro/SSA tests. Since the etiology of ILD was secondary to SS, pirfenidone was discontinued, and the patient was started on steroid therapy with subsequent marked clinical improvement. Parotid biopsy revealed the presence of inflammatory cells supporting the diagnosis of ILD associated to SS. The patient was discharged home on a tapering dose of steroids. Four months after therapy with steroids, a follow-up chest CT scan without contrast showed a chronic ILD with improved ground-glass opacities (Figure 4). The patient currently is in good health without oxygen supplementation.
Discussion
Diagnosis of SS is challenging, since it may mimic other conditions such as IPF. The most common type of SS-associated ILD is nonspecific interstitial pneumonia (NSIP), although usual interstitial pneumonia (UIP) can be visualized, as in this case study. Usual interstitial pneumonia
Diagnosing IPF cannot be solely based on a lung biopsy consistent with UIP. Appropriate diagnosis should consider the clinical presentation; PFT, laboratory findings (including rheumatologic workup), imaging (especially radiographic patterns), and biopsies. Moreover, the pathologic characteristic of IPF, which is UIP, can be found with other diseases, such as SS. Thus, it is important to make an accurate diagnosis to provide the appropriate treatment available. Patients with ILD associated with SS who have worsening symptoms, PFT, and radiographic abnormalities may be treated with oral prednisone (daily dose: 1 mg/kg).
Conclusion
This case highlights the importance of making an adequate diagnosis of ILD considering that available treatments differ for all possible etiologies other than IPF. This is a true clinical concern taking into account that many patients might be receiving inappropriate therapy for IPF diagnosis, as illustrated in the case study.
1. Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren’s syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005;171(6):632-638.
2. Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25(140):110-123.
3. Koyama M, Johkoh T, Honda O, et al. Pulmonary involvement in primary Sjögren’s syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging. 2001;16(4):290-296.
4. Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014;23(133):308-319.
Sjogren syndrome (SS) is a chronic inflammatory autoimmune disorder characterized by lymphocytic infiltration of lacrimal and salivary glands causing sicca syndrome.¹ The disease can extend beyond the exocrine glands, and systemic manifestations, including vasculitis, lung, renal or neurologic involvement, can occur.² Lung disease associated with SS is more commonly seen in women aged ≥ 60 years. The most common symptoms include dry cough, chest pain, and dyspnea on exertion. Sjogren syndrome also may produce several respiratory complications, including bronchial hyperresponsiveness, bronchiolitis, bronchiectasis, pulmonary infections, pulmonary amyloidosis, pulmonary embolism, pulmonary hypertension, lymphomas, and interstitial lung diseases (ILD).² Although ILD typically occurs 5 to 10 years after the onset of SS, lung disease can precede SS.
Pulmonary involvement is associated with systemic manifestations, hypergammaglobulinemia, and anti-SSA and anti-SSB antibodies.² Laboratory tests that confirm a diagnosis of SS include antinuclear antibody (ANA), anti-Ro/SSA, and anti-La/SSB antibodies.² Pulmonary function test (PFT) results appear to reflect impairment of either the lung (restrictive syndrome) or airways (obstructive syndrome).² Imaging abnormalities may include ground-glass attenuation, subpleural small nodules, nonseptal linear opacities, interlobular septal thickening, bronchiectasis, and cysts.³ Therefore, many ILD cases show similar imaging and pathologic findings; nevertheless, they have identifiable etiology that are not idiopathic.
Case Presentation
A 67-year-old man with a medical history of hypertension, peripheral vascular disease, and keratoconjunctivitis sicca (treated with eye drops) developed progressive shortness of breath, dyspnea on exertion, and weight loss (40 pounds) over the course of 6 months. A pulmonary function test showed a restrictive abnormality with decreased diffusing capacity of the lungs for carbon monoxide (eFigure available online at www.fedprac.com). A chest computed tomography (CT) scan showed the presence of significant thickening of the interlobular septi that was more pronounced in the subpleural regions of the lungs and lower lobes, which was consistent with usual interstitial pneumonia. A chest X-ray conducted 4 months prior showed no significant acute cardiopulmonary abnormalities (Figure 1). An open lung wedge biopsy revealed chronic organizing pneumonia with mild interstitial chronic inflammation, smooth muscle hypertrophy, and honeycomb changes consistent with usual interstitial pneumonia.
The patient had been diagnosed with idiopathic pulmonary fibrosis (IPF) by a private physician and started pirfenidone and oxygen therapy. Three months later the patient presented to the VA Caribbean Healthcare System in San Juan Puerto Rico when he developed an exacerbation of IPF. The patient reported having fever, chills, dry cough, night sweats, and marked shortness of breath. He was found hypoxemic (partial pressure of O2 was 50 mm Hg) and required a venturi mask set to 50% fractioned of inspired O2 to maintain a peripheral oxygen saturation around 90%. A chest X-ray showed decreased lung volume with bilateral interstitial and alveolar disease (Figure 2). Leukocytosis was present at 17×10-3/µl. The chest CT scan showed interval worsening of diffuse ground-glass airspace opacities and worsening of interstitial opacities; there
After careful clinical assessment (+ dry eyes) and radiographic pattern evaluation (diffuse bilateral interstitial and ground-glass opacities), the clinical diagnosis of IPF was queried after the patient’s rheumatologic workup came back positive for ANA and anti-Ro/SSA tests. Since the etiology of ILD was secondary to SS, pirfenidone was discontinued, and the patient was started on steroid therapy with subsequent marked clinical improvement. Parotid biopsy revealed the presence of inflammatory cells supporting the diagnosis of ILD associated to SS. The patient was discharged home on a tapering dose of steroids. Four months after therapy with steroids, a follow-up chest CT scan without contrast showed a chronic ILD with improved ground-glass opacities (Figure 4). The patient currently is in good health without oxygen supplementation.
Discussion
Diagnosis of SS is challenging, since it may mimic other conditions such as IPF. The most common type of SS-associated ILD is nonspecific interstitial pneumonia (NSIP), although usual interstitial pneumonia (UIP) can be visualized, as in this case study. Usual interstitial pneumonia
Diagnosing IPF cannot be solely based on a lung biopsy consistent with UIP. Appropriate diagnosis should consider the clinical presentation; PFT, laboratory findings (including rheumatologic workup), imaging (especially radiographic patterns), and biopsies. Moreover, the pathologic characteristic of IPF, which is UIP, can be found with other diseases, such as SS. Thus, it is important to make an accurate diagnosis to provide the appropriate treatment available. Patients with ILD associated with SS who have worsening symptoms, PFT, and radiographic abnormalities may be treated with oral prednisone (daily dose: 1 mg/kg).
Conclusion
This case highlights the importance of making an adequate diagnosis of ILD considering that available treatments differ for all possible etiologies other than IPF. This is a true clinical concern taking into account that many patients might be receiving inappropriate therapy for IPF diagnosis, as illustrated in the case study.
Sjogren syndrome (SS) is a chronic inflammatory autoimmune disorder characterized by lymphocytic infiltration of lacrimal and salivary glands causing sicca syndrome.¹ The disease can extend beyond the exocrine glands, and systemic manifestations, including vasculitis, lung, renal or neurologic involvement, can occur.² Lung disease associated with SS is more commonly seen in women aged ≥ 60 years. The most common symptoms include dry cough, chest pain, and dyspnea on exertion. Sjogren syndrome also may produce several respiratory complications, including bronchial hyperresponsiveness, bronchiolitis, bronchiectasis, pulmonary infections, pulmonary amyloidosis, pulmonary embolism, pulmonary hypertension, lymphomas, and interstitial lung diseases (ILD).² Although ILD typically occurs 5 to 10 years after the onset of SS, lung disease can precede SS.
Pulmonary involvement is associated with systemic manifestations, hypergammaglobulinemia, and anti-SSA and anti-SSB antibodies.² Laboratory tests that confirm a diagnosis of SS include antinuclear antibody (ANA), anti-Ro/SSA, and anti-La/SSB antibodies.² Pulmonary function test (PFT) results appear to reflect impairment of either the lung (restrictive syndrome) or airways (obstructive syndrome).² Imaging abnormalities may include ground-glass attenuation, subpleural small nodules, nonseptal linear opacities, interlobular septal thickening, bronchiectasis, and cysts.³ Therefore, many ILD cases show similar imaging and pathologic findings; nevertheless, they have identifiable etiology that are not idiopathic.
Case Presentation
A 67-year-old man with a medical history of hypertension, peripheral vascular disease, and keratoconjunctivitis sicca (treated with eye drops) developed progressive shortness of breath, dyspnea on exertion, and weight loss (40 pounds) over the course of 6 months. A pulmonary function test showed a restrictive abnormality with decreased diffusing capacity of the lungs for carbon monoxide (eFigure available online at www.fedprac.com). A chest computed tomography (CT) scan showed the presence of significant thickening of the interlobular septi that was more pronounced in the subpleural regions of the lungs and lower lobes, which was consistent with usual interstitial pneumonia. A chest X-ray conducted 4 months prior showed no significant acute cardiopulmonary abnormalities (Figure 1). An open lung wedge biopsy revealed chronic organizing pneumonia with mild interstitial chronic inflammation, smooth muscle hypertrophy, and honeycomb changes consistent with usual interstitial pneumonia.
The patient had been diagnosed with idiopathic pulmonary fibrosis (IPF) by a private physician and started pirfenidone and oxygen therapy. Three months later the patient presented to the VA Caribbean Healthcare System in San Juan Puerto Rico when he developed an exacerbation of IPF. The patient reported having fever, chills, dry cough, night sweats, and marked shortness of breath. He was found hypoxemic (partial pressure of O2 was 50 mm Hg) and required a venturi mask set to 50% fractioned of inspired O2 to maintain a peripheral oxygen saturation around 90%. A chest X-ray showed decreased lung volume with bilateral interstitial and alveolar disease (Figure 2). Leukocytosis was present at 17×10-3/µl. The chest CT scan showed interval worsening of diffuse ground-glass airspace opacities and worsening of interstitial opacities; there
After careful clinical assessment (+ dry eyes) and radiographic pattern evaluation (diffuse bilateral interstitial and ground-glass opacities), the clinical diagnosis of IPF was queried after the patient’s rheumatologic workup came back positive for ANA and anti-Ro/SSA tests. Since the etiology of ILD was secondary to SS, pirfenidone was discontinued, and the patient was started on steroid therapy with subsequent marked clinical improvement. Parotid biopsy revealed the presence of inflammatory cells supporting the diagnosis of ILD associated to SS. The patient was discharged home on a tapering dose of steroids. Four months after therapy with steroids, a follow-up chest CT scan without contrast showed a chronic ILD with improved ground-glass opacities (Figure 4). The patient currently is in good health without oxygen supplementation.
Discussion
Diagnosis of SS is challenging, since it may mimic other conditions such as IPF. The most common type of SS-associated ILD is nonspecific interstitial pneumonia (NSIP), although usual interstitial pneumonia (UIP) can be visualized, as in this case study. Usual interstitial pneumonia
Diagnosing IPF cannot be solely based on a lung biopsy consistent with UIP. Appropriate diagnosis should consider the clinical presentation; PFT, laboratory findings (including rheumatologic workup), imaging (especially radiographic patterns), and biopsies. Moreover, the pathologic characteristic of IPF, which is UIP, can be found with other diseases, such as SS. Thus, it is important to make an accurate diagnosis to provide the appropriate treatment available. Patients with ILD associated with SS who have worsening symptoms, PFT, and radiographic abnormalities may be treated with oral prednisone (daily dose: 1 mg/kg).
Conclusion
This case highlights the importance of making an adequate diagnosis of ILD considering that available treatments differ for all possible etiologies other than IPF. This is a true clinical concern taking into account that many patients might be receiving inappropriate therapy for IPF diagnosis, as illustrated in the case study.
1. Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren’s syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005;171(6):632-638.
2. Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25(140):110-123.
3. Koyama M, Johkoh T, Honda O, et al. Pulmonary involvement in primary Sjögren’s syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging. 2001;16(4):290-296.
4. Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014;23(133):308-319.
1. Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren’s syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005;171(6):632-638.
2. Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25(140):110-123.
3. Koyama M, Johkoh T, Honda O, et al. Pulmonary involvement in primary Sjögren’s syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging. 2001;16(4):290-296.
4. Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014;23(133):308-319.