Article
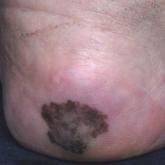
Irregularly Hyperpigmented Plaque on the Right Heel
- Author:
- Ji Young Yang, MD
- Soo Yeon Cho, MD
- You Chan Kim, MD, PhD
A 56-year-old woman presented with an asymptomatic plaque on the right heel that had grown
steadily over the last year. Pigmented lesions...
Article
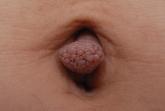
What Is Your Diagnosis? Acquired Lymphangiectasia
- Author:
- Dong Jun Lee, MD
- Soo-Eun Jung, MD
- You Chan Kim, MD, PhD
A 19-year-old woman presented with an umbilical mass of 5 months’ duration that had grown in size. Physical examination revealed a 1×1-cm brownish...
Article
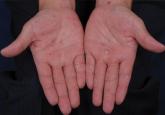
Atypical Presentation of Secondary Syphilis
- Author:
- Dong Jun Lee, MD
- You Chan Kim, MD, PhD
Syphilis is caused by Treponema pallidum and clinically presents with variable mucocutaneous features. The clinical features of secondary syphilis...
