User login
A Lifetime in the Making
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
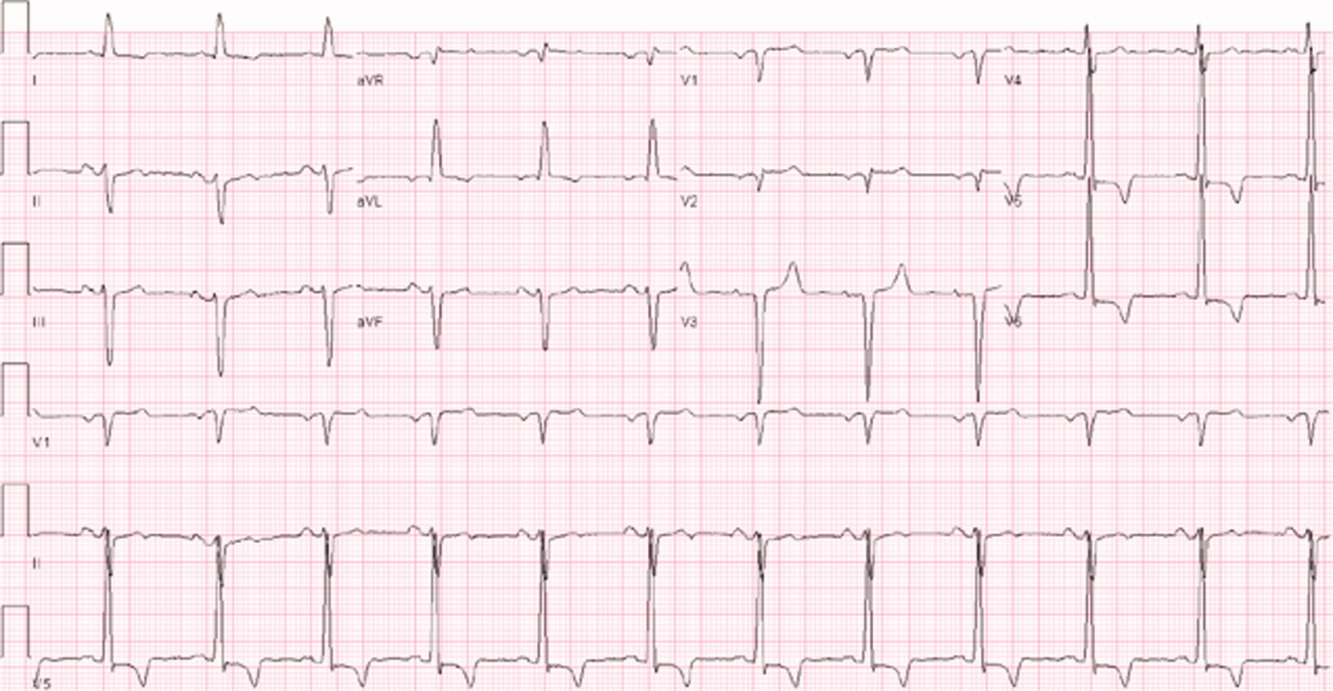
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.
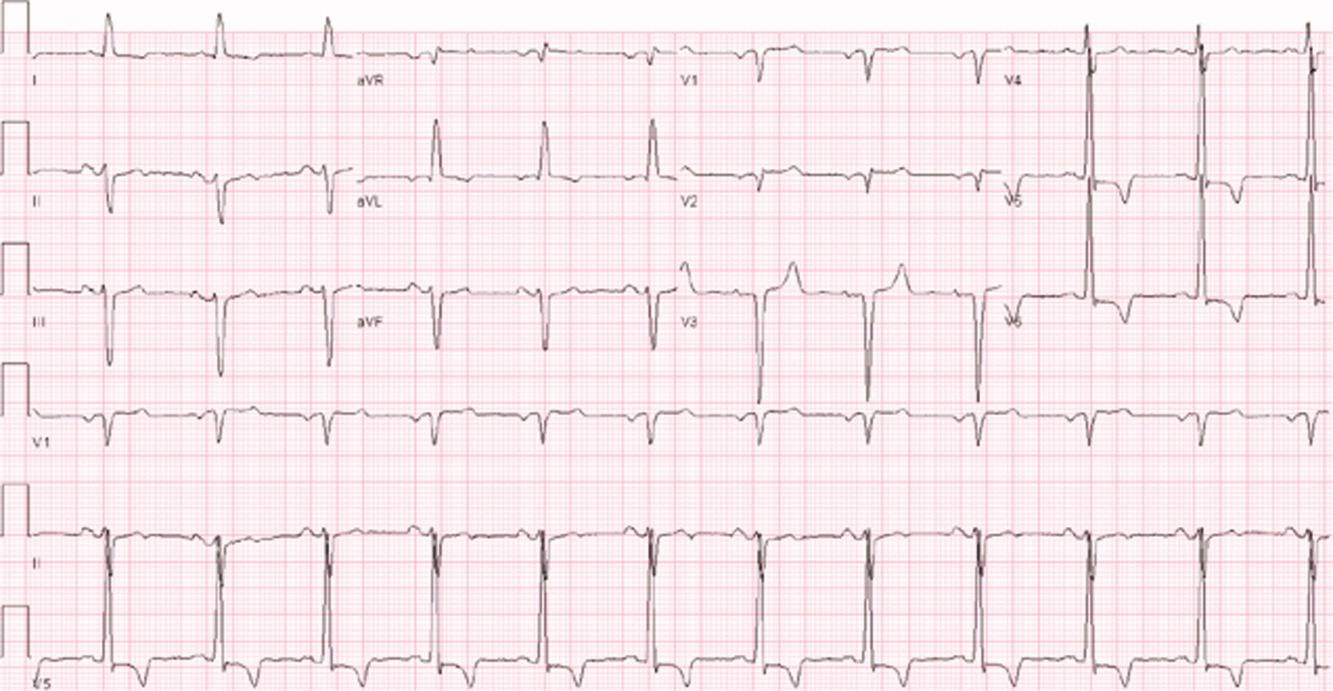
The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).
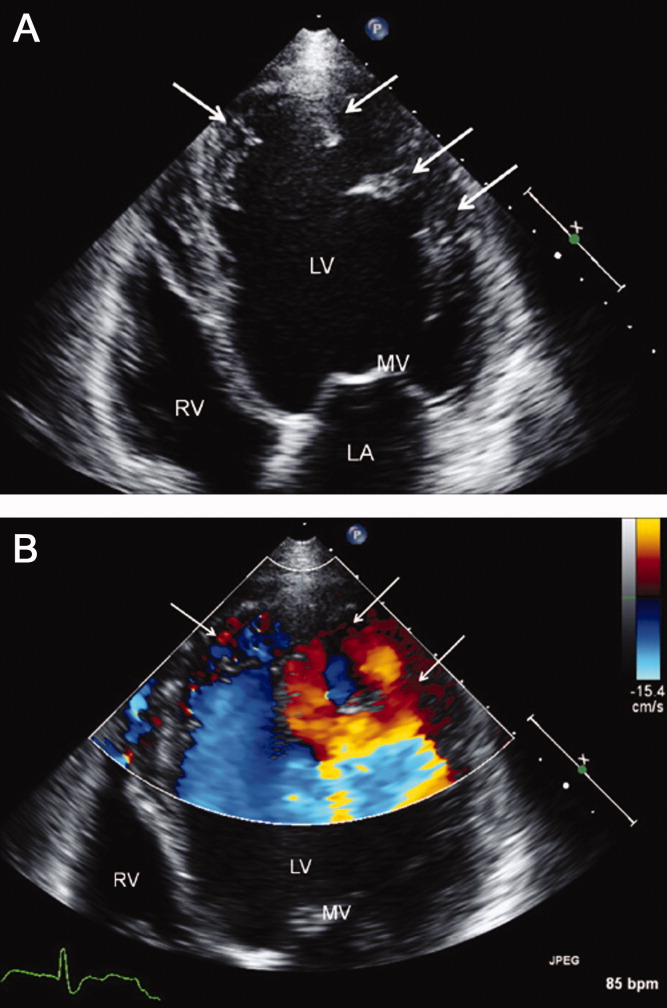
The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.

The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).

The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.

The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).

The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.