News
BRUISE CONTROL: Continue warfarin during cardiac device surgery
Major finding: The incidence of clinically significant device-pocket hematoma in patients at high thromboembolic risk who underwent pacemaker or...

AT THE AHA SCIENTIFIC SESSIONS
ANAHEIM, CALIF. – Whether direct oral anticoagulants are continued or interrupted for device placement in atrial fibrillation patients, the risk of device pocket hematoma or stroke is very low, based on results of the BRUISE CONTROL–2 trial in more than 600 subjects.
Either strategy is reasonable depending on the clinical scenario, coprincipal investigator David Birnie, MD, said in presenting the results at the American Heart Association scientific sessions.
When atrial fibrillation (AF) patients on direct oral anticoagulants (DOACs) present for device surgery, there’s concern that keeping them on the drugs will increase the bleeding risk, but that taking them off will increase the stroke risk. “We sought to resolve this dilemma,” said Dr. Birnie, an electrophysiologist and director of the arrhythmia service at the University of Ottawa Heart Institute.
The subjects were on dabigatran, rivaroxaban, or apixaban, about a third in each group; 328 were randomized to continue their daily dosing, including on the day of surgery. The other 334 were randomized to interrupted treatment. For rivaroxaban and apixaban, that meant taking their last dose 2 days before surgery. Dabigatran patients discontinued the drug 1-2 days beforehand, depending on glomerular filtration rate. Patients resumed treatment about 24 hours after surgery. CHA2DS2-VASc scores were a mean of 3.9 in both arms, and at least 2 in all participants.
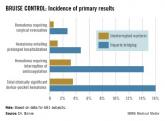
The rate of clinically significant hematoma – the primary outcome in the study, defined as a hematoma requiring prolonged hospitalization, interrupted postoperative anticoagulation, or reoperation to evacuate – was identical in both arms, 2.1% (seven patients each). There were two ischemic strokes, one in each arm. There was one delayed cardiac tamponade in the continuation arm and one pericardial effusion in the interrupted arm. The three deaths in the trial were not related to device placement.
So, what to do depends on the clinical scenario, Dr. Birnie said in an interview. If someone needs urgent placement and there’s no time to wait for DOAC washout, “it’s quite reasonable to go ahead.” Also, “if somebody is at extremely high risk for stroke, then it’s very reasonable to continue the drug.”
On the other hand, “if someone has a much lower stroke risk, then the risk-benefit ratio is probably in the opposite direction, so temporarily discontinuing the drug is the right thing to do,” he said.
Dr. Birnie cautioned that although continued DOAC may reduce the risk of thromboembolism, “this study was not designed with power to answer this.”
“We are already putting these findings into practice” in Ottawa, he said. “Our protocol” – as in many places – “ was always to stop anticoagulation for 2 or 3 days, but now, for very high-risk patients – high-risk AF, unstable temporary pacing, that type of thing – we are very comfortable continuing it,” he said. The study follows up a previous randomized trial by Dr. Birnie and his colleagues that pitted continued warfarin against heparin bridging for AF device placement. There were far fewer device pocket hematomas with uninterrupted warfarin (N Engl J Med. 2013 May 30;368[22]:2084-93).
The team wanted to repeat the study using DOACs, since their use has grown substantially, with the majority of AF patients now on them.
The arms in BRUISE CONTROL–2 (Strategy of Continued Versus Interrupted Novel Oral Anticoagulant at Time of Device Surgery in Patients With Moderate to High Risk of Arterial Thromboembolic Events) were well matched, with a mean age of about 74 years; men made up more than 70% of the subjects in both arms. About 17% of the participants were on chronic aspirin therapy and about 4% were on clopidogrel, in each arm. The uninterrupted DOAC group went about 14 hours between their last preop and first postop DOAC dose. The interrupted group went about 72 hours.
BRUISE CONTROL–2 was funded by the Heart and Stroke Foundation of Canada, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb, among others. Dr. Birnie had no relevant financial disclosures.

Major finding: The incidence of clinically significant device-pocket hematoma in patients at high thromboembolic risk who underwent pacemaker or...
