Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
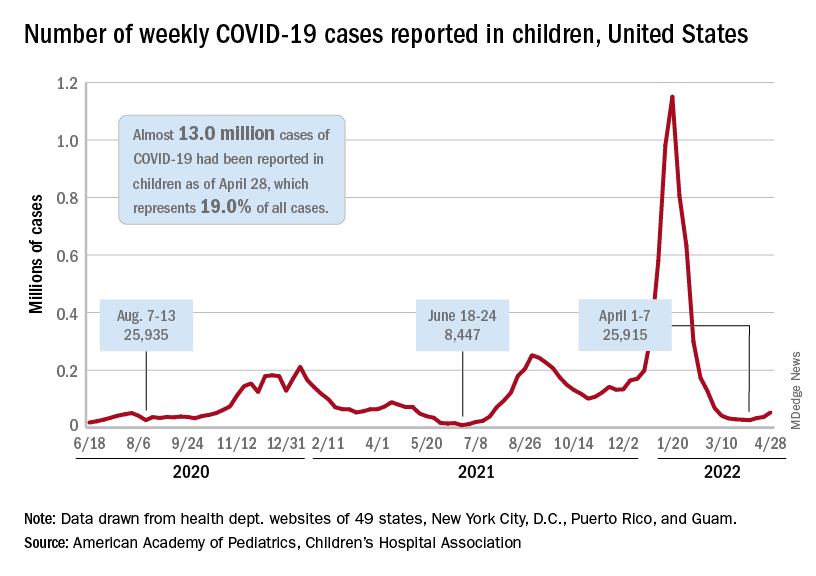
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
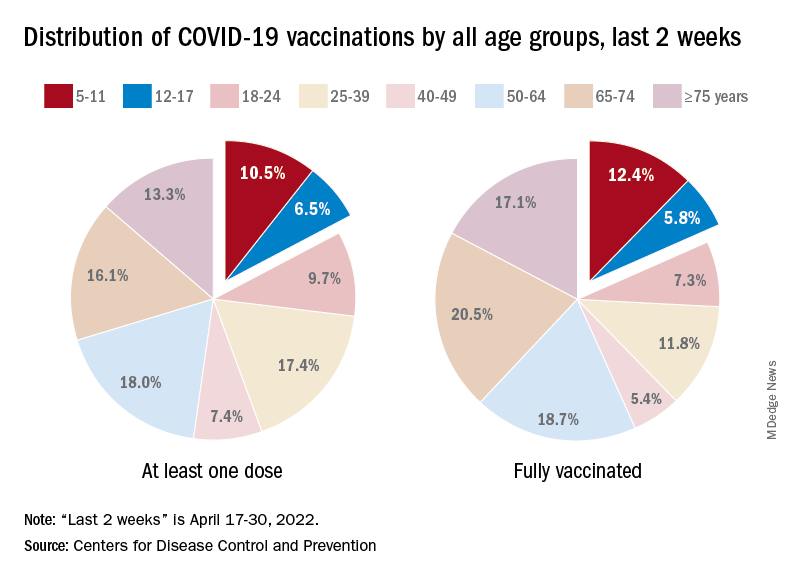
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.