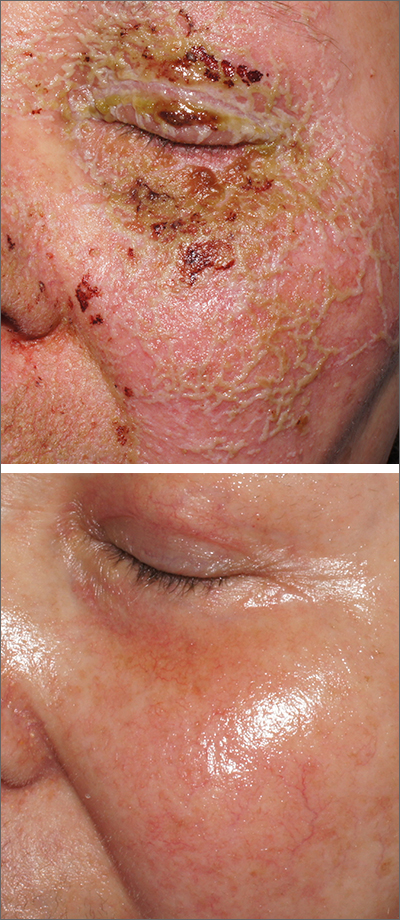
A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.