Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
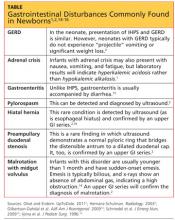
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17