The immune response to a live, attenuated vaccine is virtually identical to a response produced by natural infection. In rare instances, however, live, attenuated vaccines may cause severe or fatal reactions as a result of uncontrolled replication of the organism. This occurs only in individuals who are significantly immunocompromised.10,16
Inactivated vaccines are produced by growing the viral or bacterial organism in a culture medium, then using heat and/or chemicals to inactivate the organism. Because inactivated vaccines are not alive, they cannot replicate, and therefore cannot cause disease, even in an immunodeficient patient. The immune response to an inactivated vaccine is mostly humoral (in contrast to the natural infection response of a live vaccine), and little or no cellular immunity is produced.10,16
Inactivated vaccines always require multiple doses, gradually building up a protective immune response. The antibody titers diminish over time, so some inactivated vaccines may require periodic doses to “boost” or increase the titers.16
Some vaccines, such as hepatitis B vaccine, lead to the development of immune memory, which stays intact for at least 20 years following immunization. Immune memory occurs during replication of B cells and T cells; some cells will become long-lived memory cells that will “remember” the pathogen and produce an immune response if the pathogen is detected again. In this case, boosters are not recommended.10
Toxoids are a type of vaccine made from the inactivated toxin of a bacterium—not the bacterium itself. Tetanus and diphtheria vaccines are examples of toxoid vaccines.10,16
Subunit and conjugate vaccines are segments of the pathogen. A subunit vaccine can be created via genetic engineering. The end result is a recombinant vaccine that can stimulate cell memory (eg, hepatitis B vaccine).10,16 Conjugate vaccines, which are similar to recombinant vaccines, are made by combining two different components to prompt a more powerful, combined immune response.10
Spacing of Live-Virus and Inactivated Vaccines
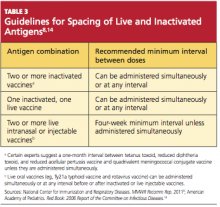
There are almost no spacing requirements between two or more inactivated vaccines8 (see Table 38,14 for spacing recommendations from the Advisory Committee on Immunization Practices [ACIP]). The only vaccines that must be spaced at least four weeks apart are live-virus vaccines—that is, if they are not given on the same day. Studies have shown that the immune response to a live-virus vaccine may be impaired if it is administered within 30 days of another live-virus vaccine. Inactivated vaccines, on the other hand, may usually be administered at any time after or before a live-virus vaccine.8
One exception to this statement is the administration of Zostavax (zoster live-virus vaccine) with Pneumovax 23 (inactivated pneumococcal vaccine, polyvalent, MSD).17-19 The manufacturer of the two vaccines recommends a spacing of at least four weeks between them, based on research showing that concomitant use may result in reduced immunogenicity for Zostavax.20 However, as of this writing, ACIP has not revised its statement that both vaccines can be given at the same time or at any time before or after each other.21
Live-virus vaccines currently licensed in the US provide protection against diseases including measles/mumps/rubella, varicella, zoster (ie, shingles), influenza, and yellow fever.
ADULT VACCINATION HIGHLIGHTS
A summary of 2011 recommendations for adult immunization from ACIP is shown in Table 412,21. The following information is specific to each of the vaccine-preventable illnesses of concern in adults.12,13,22
Seasonal Influenza
The options to protect the adult patient against seasonal influenza are a trivalent, inactivated influenza vaccine (TIV; Fluzone, high-dose Fluzone for adults ages 65 and older, Fluvirin, Fluarix, FluLaval, Afluria, Agriflu) or a live, attenuated influenza vaccine (LAIV; FluMist).2,23 Dosage of TIV for adults is 0.5 mL IM in the deltoid once annually. For adults ages 49 and younger, LAIV is administered at 0.2 mL intranasally, once per year.
ACIP now recommends universal influenza vaccination for all persons ages 6 months and older with no contraindications.2,8 Strong consideration should be given to concurrent administration of influenza vaccine and pneumococcal vaccine to high-risk persons not previously vaccinated against pneumococcal disease.12
Note: When influenza and pneumococcal vaccines are given at the same time, they should be administered in opposite arms to reduce the risk of adverse reactions or a decreased antibody response to either vaccine.18,19
Pneumococcal Polysaccharide (PPSV)
Pneumovax 2319 is administered as a 0.5-mL dose IM in the deltoid or subcutaneously in the upper arm. The vaccine is recommended for8,19,24:
• Adults ages 65 and older who have not been previously vaccinated
• Adults now 65 and older who received PPSV vaccine at least five years ago and were younger than 65 at that time
• Adults ages 19 through 64 years who have asthma or who smoke24