User login
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
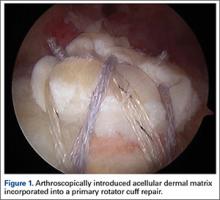
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
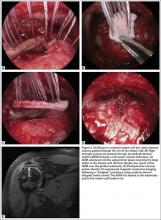
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
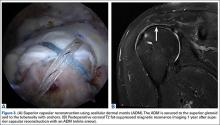
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.