User login
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
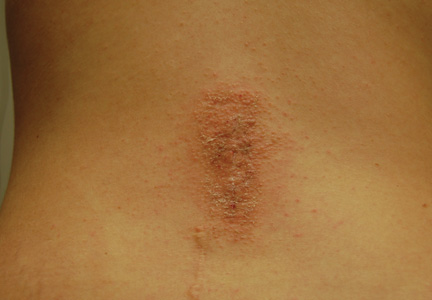
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 | 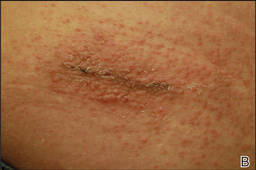 |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
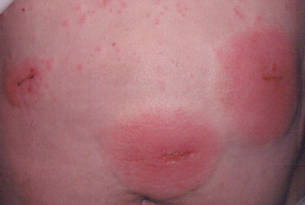 |
| Figure 2. Acute eczematous plaques at wound closures. |
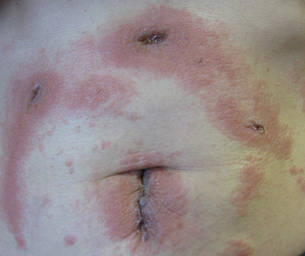 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
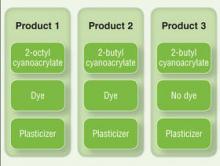
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 |  |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
 |
| Figure 2. Acute eczematous plaques at wound closures. |
 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.
 |  |
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
 |
| Figure 2. Acute eczematous plaques at wound closures. |
 |
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.