User login
A previously healthy 11‐year‐old boy presented to the emergency department after referral from his pediatrician for 1 week of fevers. Seven days prior to admission he developed a fever to 40.8C, vomiting, and mild left knee pain. The vomiting resolved within 2 days. Five days prior to admission he developed a pruritic, pinpoint rash over his abdomen that resolved within 24 hours. He also developed red, cracked lips, redness of his tongue, redness surrounding his eyes, and slight swelling of his hands. Three days prior to admission his pediatrician noted a 1‐cm anterior cervical lymph node. His fevers occurred throughout each of the prior 7 days without a discernible pattern, and his mild knee pain persisted at the time of presentation.
This preteen has had high fevers for 1 week associated with arthralgia, pruritic rash, emesis, and oral mucosal erythema. His rash, lip and tongue erythema, and swollen hands are classic features of Kawasaki disease (KD), but he lacks the other characteristic physical examination findings. The diagnosis of KD requires fever for at least 5 days accompanied by 4 of the following 5 signs: polymorphous rash, oral mucous membrane changes, peripheral extremity changes such as swelling or skin desquamation, bilateral bulbar conjunctival injection, and cervical lymphadenopathy >1.5 cm in diameter. Children meeting fewer than 4 of these criteria may have an incomplete form of KD.
Because most patients with KD (80%) are under 5 years old, alternative diagnoses such as autoimmune illnesses or a hypersensitivity reaction should be considered. Travel, medication, and animal exposure histories may reveal clues to an infectious or drug‐induced etiology of his fever. Immunization status should be assessed, as measles is also associated with fever, rash, and mucosal changes. Arthralgia or arthritis may occur in KD, but these findings suggest the need to entertain other possibilities, including bone or joint infection, infective endocarditis, inflammatory bowel disease, juvenile idiopathic arthritis (JIA), or systemic lupus erythematosus (SLE).
The child's only past medical history was an episode of croup as an infant. There was no family history of autoimmune diseases. He was not taking any medications and had no known allergies. His immunizations were up to date, including measles, mumps, rubella, and varicella. He lived with his parents and his dog. He swam in fresh water during a trip to Maine 2 months earlier. Neither he nor his family recalled a tick bite. He had no exposure to raw meat or unpasteurized dairy products.
The travel to New England raises the possibility of Lyme disease, although a 2‐month interval between exposure and a high, prolonged fever would be very unusual. Knee arthralgia or arthritis is common in children with late‐stage Lyme disease, but can also be seen in early‐disseminated disease. The prior description of the rash is not suggestive of erythema chronicum migrans, which is seen in early‐stage Lyme disease.
C‐reactive protein (CRP) was 189 mg/L (normal <6.3 mg/L). An echocardiogram was normal. Intravenous immunoglobulin (IVIG) was administered for presumed KD, with immediate improvement of the periorbital erythema, tongue redness, and hand swelling. He was discharged the next day on aspirin with cardiology clinic follow‐up.
Improvement after IVIG supports the diagnosis of KD. It is typical to discharge KD patients from the hospital when they have been afebrile for 24 hours or when the CRP level has declined by approximately 50%.
Over the next 48 hours he felt unwell with high‐grade fevers, continued left knee pain, and new left hip pain. He was readmitted to the hospital. His temperature was 39.4C, respiratory rate was 22 breaths per minute, heart rate was 122 beats per minute, blood pressure was 103/50 mm Hg, and oxygen saturation was 100% while breathing ambient air. He appeared mildly uncomfortable. His conjunctivae were normal. His lips were dry, red, and cracked, and his tongue was red with prominent papillae. His neck was supple without lymphadenopathy. His lungs were clear to auscultation. His heart exam was without murmurs. His abdomen was soft, and the liver and spleen were not enlarged. He had no swelling or erythema of his joints; however, he experienced pain with range of motion of his left knee, and tenderness and restricted range of motion of his left hip. His neurologic exam was normal. There were no rashes.
He has persistent fever, tachycardia, and tachypnea, now without features of KD except oral mucosal changes including prominent tongue papillae consistent with a strawberry tongue. Continued or recurrent fever may suggest persistent KD with ongoing inflammation or the need to search for an alternative diagnoses. An echocardiogram should be repeated, as the coronary artery abnormalities in KD can evolve rapidly, particularly when inflammation persists. Additional findings may include decreased left ventricular function, mitral regurgitation, or pericardial effusion. A second dose of IVIG is necessary to control fever and inflammation in about 15% of patients with KD, although in this case IVIG should be withheld pending further evaluation.
Arthralgia occurs commonly in KD, whereas frank arthritis is less typical. Polyarticular or oligoarticular arthritis involving small or large joints (especially knee or ankle) affects 5% to 10% of patients. The severity of findings in his left hip warrants consideration of septic arthritis with pain referred to the knee; pelvic or femoral osteomyelitis; psoas abscess; or pyomyositis. Following basic lab tests, imaging of the left hip region is indicated.
Laboratory evaluation revealed: white blood cell (WBC) count 10,000/L (absolute neutrophil count 8,460/L, absolute lymphocyte count 530/L), hemoglobin 10.6 g/dL, platelet count 208,000/L, serum sodium 130 mmol/L, serum potassium 3.3 mmol/L, serum urea nitrogen 11 mg/dL, serum creatinine 0.54 mg/dL, aspartate transaminase (AST) 26 U/L, alanine transaminase (ALT) 31 U/L, albumin 1.7 g/dL, erythrocyte sedimentation rate (ESR) > 100 mm/h, and CRP 263 mg/L. No blast cells were seen on peripheral blood smear.
Hypoalbuminemia and markedly elevated inflammatory markers indicate an inflammatory condition that has been active for more than a week. Assessing ESR after IVIG therapy is not useful because exogenous globulins increase the ESR; however, CRP is useful to monitor inflammation and remains elevated here.
Incomplete KD is still possible. Hyponatremia, hypoalbuminemia, and anemia are all features of persistent KD, and have been utilized in several clinical scoring systems in Japan to identify KD patients at increased risk for developing coronary complications. A neoplastic process cannot be excluded, but does not appear likely based on the acuity of his presentation and peripheral blood smear review.
Upon readmission he received a second dose of 2 g/kg IVIG. He remained on aspirin and continued to have fevers. A repeat echocardiogram was normal. He had worsening pain in his left knee and hip with difficulty straightening his left leg. Physical examination was notable for tenderness to palpation over his left hip joint, refusal to bear weight, and resistance to passive range of motion. On hospital day 2, an ultrasound of his left hip and knee revealed a complex left hip effusion and small left knee effusion.
KD becomes less likely in the presence of persistent fevers after IVIG and a repeatedly normal echocardiogram. Worsening left leg symptoms including impaired hip extension with a complex hip effusion suggests an infectious process in or adjacent to the left hip, such as septic arthritis, myositis, or osteomyelitis of the pelvis or proximal femur. A complex hip effusion is less likely to be present with arthritis related to JIA or SLE. The patient needs an emergent hip aspiration and possibly magnetic resonance imaging (MRI) to evaluate adjacent structures.
Arthrotomy and open drainage of his left hip revealed purulent fluid with a WBC count of 49,000/L with 89% neutrophils and 2% lymphocytes. Gram stain was negative. A left knee aspirate demonstrated straw‐colored synovial fluid (which was not sent for cell counts). Bacterial, fungal, and acid‐fast bacilli cultures were requested from hip and knee aspirates. Intravenous ceftriaxone and vancomycin were administered.
The most likely organism in pediatric pyogenic arthritis is Staphylococcus aureus, but there is a long list of other potential pathogens, including Streptococcus pyogenes (group A streptococcus) and Streptococcus pneumoniae. Most pediatric patients with acute pyogenic arthritis have synovial fluid WBC counts in excess of 75,000 to 100,000/L. The protracted course and the initial lack of hip symptoms raise the possibility of a primary osteomyelitis of the femur (particularly the intracapsular portion of the femoral neck or head) or of the acetabulum, with subsequent extension into the hip joint. Pyogenic myositis involving muscle groups adjacent to the hip would be unlikely to spread into the hip space, but can lead to synovial irritation, characterized by sterile joint fluid and WBC counts that fall short of the usual numbers seen in septic arthritis. The blood supply to the femoral head can become compromised with prolonged inflammation and increased intracapsular pressure, resulting in aseptic necrosis.
All cultures from his hip and knee aspirations were sterile. He continued to have daily fevers and persistent tachycardia while receiving intravenous ceftriaxone and vancomycin. Additional testing was notable for: antinuclear antibody (ANA) 1:80, anti‐streptolysin O (ASO) titer 344 IU (normal <150 IU), AST and ALT within normal limits, ferritin 568 ng/mL (normal <322 ng/mL), and lactate dehydrogenase (LDH) 212 units/L (normal <257 units/L). Abdominal ultrasound revealed borderline hepatosplenomegaly. An ophthalmologic examination was normal.
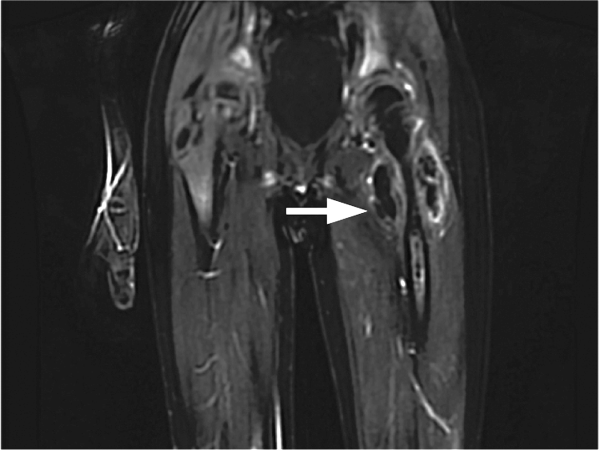
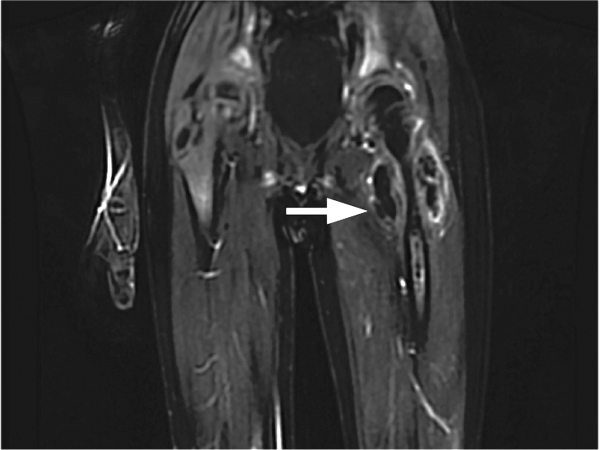
On postoperative day 4 he developed left upper thigh swelling. An MRI showed rim‐enhancing juxta‐articular complex fluid collections surrounding the left femur with decreased marrow enhancement of the left proximal femur (Figure 1).
The limited rheumatologic evaluation is unrevealing; the ANA result is nondiagnostic and the ASO titer is normal for age. Laboratories generally report adult normal values for streptococcal antibodies regardless of the patient's age; children from ages 7 to 12 years are at their life peak frequency of group A streptococcal pharyngitis and typically have higher normal values of streptococcal antibodies, including ASO (up to about 480640 IU). The moderately elevated ferritin level is most likely an acute phase reactant and not high enough to suggest macrophage activation syndrome, which is unlikely with the normal AST, ALT, and LDH levels, the absence of significant splenomegaly, and the lack of cytopenias. Continued fever with progressive left upper thigh swelling point to osteomyelitis of the proximal femur, which may have initially ruptured into the hip and then infiltrated the femoral cortex and spread infection into the adjacent soft tissues. Surgical debridement is indicated.
The relative prevalence of methicillin‐sensitive S aureus (MSSA) and methicillin‐resistant S aureus vary widely with geography. MSSA strains are more likely to be highly toxigenic. The elaboration of 1 or more extracellular toxins could account for the patient's initial symptoms.
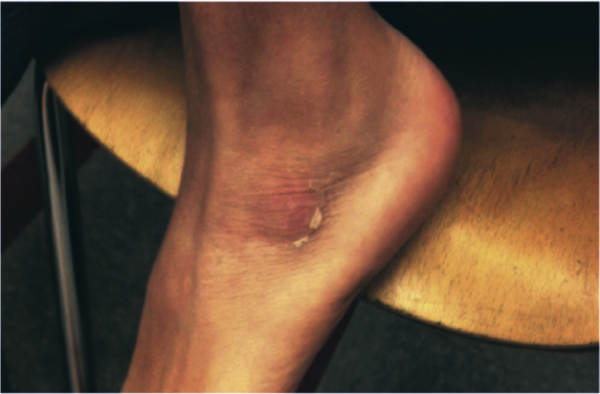
The patient was brought back to the operating room for drainage of the juxta‐articular fluid collections and a biopsy of his femur. The fluid collections were grossly purulent. His intraoperative cultures were positive for MSSA. The bone biopsy revealed necrotic tissue, acute inflammation, and bacterial colonies, consistent with acute osteomyelitis. Further testing of his S aureus isolate was positive for staphylococcal enterotoxin B. He completed a 4‐week course of oral clindamycin with subsequent normalization of his hip exam and inflammatory markers. At a follow‐up visit the patient was feeling better, but had developed skin peeling on the lateral aspects of his feet consistent with late sequelae of toxin‐mediated disease (Figure 2). Three months after discharge the patient had returned to his baseline activity level and remained asymptomatic.
COMMENTARY
The patient presented with a constellation of symptoms that was initially mistaken for incomplete KD until focal progression of his symptoms exposed an underlying femoral osteomyelitis with periarticular abscess formation. Bacterial cultures and subsequent toxin assay revealed an enterotoxin B‐producing strain of S aureus.
Certain staphylococcal strains secrete superantigens that may lead to the development of a systemic toxin‐mediated syndrome. Toxins elaborated by S aureus include toxic shock syndrome toxin‐1 (TSST‐1) and enterotoxins, which have been implicated in menstrual and nonmenstrual toxic shock syndromes.[1, 2] Enterotoxin B is a staphylococcal superantigen found in most strains of the USA400 clonal group, and has been frequently associated with skin and soft tissue infections.[3] Enterotoxin B production has been reported in nearly half of S aureus isolates from skin, soft tissue, and bone infections.[4]
Staphylococcal and streptococcal toxin‐mediated diseases can mimic vasculitis, systemic juvenile idiopathic arthritis, viral infections, and Stevens‐Johnson syndrome. Glossitis in toxin‐mediated syndromes manifests with a swollen, red tongue with overlying enlarged papillae, giving the appearance of a strawberry. Although pediatric providers often equate strawberry tongue, conjunctival injection, rash, and erythematous lips with KD, these findings are also seen in toxin‐mediated diseases, such as scarlet fever or staphylococcal toxic shock syndrome. Enterotoxin B mediated staphylococcal disease masquerading as KD has been reported in 2 cases: a 7‐month‐old boy with multifocal S aureus osteomyelitis and a 5‐year‐old boy with S aureus bacteremia. Both staphylococcal isolates produced enterotoxin B but were negative for other staphylococcus‐related toxins including TSST‐1.[5]
The Institute of Medicine (IOM) recently released its report Improving Diagnosis in Health Care, highlighting the under‐recognized quality and safety issue of diagnostic error.[6] The report uses the following broad and patient‐centered definition of diagnostic error: the failure to (a) establish an accurate and timely explanation of the patient's health problem(s) or (b) communicate that explanation to the patient. The IOM's conceptual model of diagnosis emphasizes the iterative nature of the diagnostic process, including the importance of generating a working diagnosis, gathering and incorporating new information in the reassessment of that diagnosis, and integrating treatment response into the formulation of the final diagnosis (Figure 3).

Even though the patient initially had several features consistent with KD, the increasing number of atypical features could have prompted the clinical team to reconsider their working diagnosis. The patient's age was atypical for KD, he had progressive knee and hip arthritis, and his fevers persisted after IVIG. An expanded differential should have included toxic shock syndrome; the resolution of conjunctival and mucosal injection and edema after IVIG may have been the result of antibodies in the IVIG preparation with neutralizing activity against superantigens. This antitoxin activity has established a role for IVIG in the management of staphylococcal toxic shock syndrome.[7] Ultimately, his imaging and surgical drainage revealed a focal staphylococcal toxin‐producing infectious source from which his fevers, rash, and mucosal and extremity changes emanated. This case reminds us that the more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should take it for another spin around the diagnostic wheel in search of a more suitable alternative.
KEY LEARNING POINTS
- Staphylococcal toxin‐mediated disease may mimic KD, with common features including strawberry tongue, oral and conjunctival injection, and skin desquamation.
- Improvement after treatment with IVIG is characteristic but not diagnostic of KD, and may be seen in toxin‐mediated disease.
- KD may present with arthralgia or arthritis, but severe joint abnormalities warrant consideration of infectious and other autoimmune conditions.
- The more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should gather, interpret, and integrate new information in search of a more suitable alternative.
Disclosure: Nothing to report.
- . Staphylococcal enterotoxin B and toxic shock syndrome toxin‐1 are significantly associated with non‐menstrual TSS. Lancet. 1986;1:1149–1150.
- , , , , . Toxic shock syndrome caused by a strain of staphylococcus aureus that produces enterotoxin C but not toxic shock syndrome toxin‐1. Am J Dis Child. 1989;143 (7):848–849.
- , , , , , . Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS One. 2012;7(7):e41157.
- , , , et al. Variability of antibiotic susceptibility and toxin production of Staphylococcal aureus stains isolated from skin, soft tissue, and bone related infections. BMC Microbiol. 2013;13:188.
- , , , , . Kawasaki syndrome‐like illness associated with infection caused by enterotoxin B‐secreting Staphylococcus aureus. Clin Microbiol Rev. 2013;26:422–447.
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015.
- , , , et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clin Infect Dis. 2003;37(3):333–340.
A previously healthy 11‐year‐old boy presented to the emergency department after referral from his pediatrician for 1 week of fevers. Seven days prior to admission he developed a fever to 40.8C, vomiting, and mild left knee pain. The vomiting resolved within 2 days. Five days prior to admission he developed a pruritic, pinpoint rash over his abdomen that resolved within 24 hours. He also developed red, cracked lips, redness of his tongue, redness surrounding his eyes, and slight swelling of his hands. Three days prior to admission his pediatrician noted a 1‐cm anterior cervical lymph node. His fevers occurred throughout each of the prior 7 days without a discernible pattern, and his mild knee pain persisted at the time of presentation.
This preteen has had high fevers for 1 week associated with arthralgia, pruritic rash, emesis, and oral mucosal erythema. His rash, lip and tongue erythema, and swollen hands are classic features of Kawasaki disease (KD), but he lacks the other characteristic physical examination findings. The diagnosis of KD requires fever for at least 5 days accompanied by 4 of the following 5 signs: polymorphous rash, oral mucous membrane changes, peripheral extremity changes such as swelling or skin desquamation, bilateral bulbar conjunctival injection, and cervical lymphadenopathy >1.5 cm in diameter. Children meeting fewer than 4 of these criteria may have an incomplete form of KD.
Because most patients with KD (80%) are under 5 years old, alternative diagnoses such as autoimmune illnesses or a hypersensitivity reaction should be considered. Travel, medication, and animal exposure histories may reveal clues to an infectious or drug‐induced etiology of his fever. Immunization status should be assessed, as measles is also associated with fever, rash, and mucosal changes. Arthralgia or arthritis may occur in KD, but these findings suggest the need to entertain other possibilities, including bone or joint infection, infective endocarditis, inflammatory bowel disease, juvenile idiopathic arthritis (JIA), or systemic lupus erythematosus (SLE).
The child's only past medical history was an episode of croup as an infant. There was no family history of autoimmune diseases. He was not taking any medications and had no known allergies. His immunizations were up to date, including measles, mumps, rubella, and varicella. He lived with his parents and his dog. He swam in fresh water during a trip to Maine 2 months earlier. Neither he nor his family recalled a tick bite. He had no exposure to raw meat or unpasteurized dairy products.
The travel to New England raises the possibility of Lyme disease, although a 2‐month interval between exposure and a high, prolonged fever would be very unusual. Knee arthralgia or arthritis is common in children with late‐stage Lyme disease, but can also be seen in early‐disseminated disease. The prior description of the rash is not suggestive of erythema chronicum migrans, which is seen in early‐stage Lyme disease.
C‐reactive protein (CRP) was 189 mg/L (normal <6.3 mg/L). An echocardiogram was normal. Intravenous immunoglobulin (IVIG) was administered for presumed KD, with immediate improvement of the periorbital erythema, tongue redness, and hand swelling. He was discharged the next day on aspirin with cardiology clinic follow‐up.
Improvement after IVIG supports the diagnosis of KD. It is typical to discharge KD patients from the hospital when they have been afebrile for 24 hours or when the CRP level has declined by approximately 50%.
Over the next 48 hours he felt unwell with high‐grade fevers, continued left knee pain, and new left hip pain. He was readmitted to the hospital. His temperature was 39.4C, respiratory rate was 22 breaths per minute, heart rate was 122 beats per minute, blood pressure was 103/50 mm Hg, and oxygen saturation was 100% while breathing ambient air. He appeared mildly uncomfortable. His conjunctivae were normal. His lips were dry, red, and cracked, and his tongue was red with prominent papillae. His neck was supple without lymphadenopathy. His lungs were clear to auscultation. His heart exam was without murmurs. His abdomen was soft, and the liver and spleen were not enlarged. He had no swelling or erythema of his joints; however, he experienced pain with range of motion of his left knee, and tenderness and restricted range of motion of his left hip. His neurologic exam was normal. There were no rashes.
He has persistent fever, tachycardia, and tachypnea, now without features of KD except oral mucosal changes including prominent tongue papillae consistent with a strawberry tongue. Continued or recurrent fever may suggest persistent KD with ongoing inflammation or the need to search for an alternative diagnoses. An echocardiogram should be repeated, as the coronary artery abnormalities in KD can evolve rapidly, particularly when inflammation persists. Additional findings may include decreased left ventricular function, mitral regurgitation, or pericardial effusion. A second dose of IVIG is necessary to control fever and inflammation in about 15% of patients with KD, although in this case IVIG should be withheld pending further evaluation.
Arthralgia occurs commonly in KD, whereas frank arthritis is less typical. Polyarticular or oligoarticular arthritis involving small or large joints (especially knee or ankle) affects 5% to 10% of patients. The severity of findings in his left hip warrants consideration of septic arthritis with pain referred to the knee; pelvic or femoral osteomyelitis; psoas abscess; or pyomyositis. Following basic lab tests, imaging of the left hip region is indicated.
Laboratory evaluation revealed: white blood cell (WBC) count 10,000/L (absolute neutrophil count 8,460/L, absolute lymphocyte count 530/L), hemoglobin 10.6 g/dL, platelet count 208,000/L, serum sodium 130 mmol/L, serum potassium 3.3 mmol/L, serum urea nitrogen 11 mg/dL, serum creatinine 0.54 mg/dL, aspartate transaminase (AST) 26 U/L, alanine transaminase (ALT) 31 U/L, albumin 1.7 g/dL, erythrocyte sedimentation rate (ESR) > 100 mm/h, and CRP 263 mg/L. No blast cells were seen on peripheral blood smear.
Hypoalbuminemia and markedly elevated inflammatory markers indicate an inflammatory condition that has been active for more than a week. Assessing ESR after IVIG therapy is not useful because exogenous globulins increase the ESR; however, CRP is useful to monitor inflammation and remains elevated here.
Incomplete KD is still possible. Hyponatremia, hypoalbuminemia, and anemia are all features of persistent KD, and have been utilized in several clinical scoring systems in Japan to identify KD patients at increased risk for developing coronary complications. A neoplastic process cannot be excluded, but does not appear likely based on the acuity of his presentation and peripheral blood smear review.
Upon readmission he received a second dose of 2 g/kg IVIG. He remained on aspirin and continued to have fevers. A repeat echocardiogram was normal. He had worsening pain in his left knee and hip with difficulty straightening his left leg. Physical examination was notable for tenderness to palpation over his left hip joint, refusal to bear weight, and resistance to passive range of motion. On hospital day 2, an ultrasound of his left hip and knee revealed a complex left hip effusion and small left knee effusion.
KD becomes less likely in the presence of persistent fevers after IVIG and a repeatedly normal echocardiogram. Worsening left leg symptoms including impaired hip extension with a complex hip effusion suggests an infectious process in or adjacent to the left hip, such as septic arthritis, myositis, or osteomyelitis of the pelvis or proximal femur. A complex hip effusion is less likely to be present with arthritis related to JIA or SLE. The patient needs an emergent hip aspiration and possibly magnetic resonance imaging (MRI) to evaluate adjacent structures.
Arthrotomy and open drainage of his left hip revealed purulent fluid with a WBC count of 49,000/L with 89% neutrophils and 2% lymphocytes. Gram stain was negative. A left knee aspirate demonstrated straw‐colored synovial fluid (which was not sent for cell counts). Bacterial, fungal, and acid‐fast bacilli cultures were requested from hip and knee aspirates. Intravenous ceftriaxone and vancomycin were administered.
The most likely organism in pediatric pyogenic arthritis is Staphylococcus aureus, but there is a long list of other potential pathogens, including Streptococcus pyogenes (group A streptococcus) and Streptococcus pneumoniae. Most pediatric patients with acute pyogenic arthritis have synovial fluid WBC counts in excess of 75,000 to 100,000/L. The protracted course and the initial lack of hip symptoms raise the possibility of a primary osteomyelitis of the femur (particularly the intracapsular portion of the femoral neck or head) or of the acetabulum, with subsequent extension into the hip joint. Pyogenic myositis involving muscle groups adjacent to the hip would be unlikely to spread into the hip space, but can lead to synovial irritation, characterized by sterile joint fluid and WBC counts that fall short of the usual numbers seen in septic arthritis. The blood supply to the femoral head can become compromised with prolonged inflammation and increased intracapsular pressure, resulting in aseptic necrosis.
All cultures from his hip and knee aspirations were sterile. He continued to have daily fevers and persistent tachycardia while receiving intravenous ceftriaxone and vancomycin. Additional testing was notable for: antinuclear antibody (ANA) 1:80, anti‐streptolysin O (ASO) titer 344 IU (normal <150 IU), AST and ALT within normal limits, ferritin 568 ng/mL (normal <322 ng/mL), and lactate dehydrogenase (LDH) 212 units/L (normal <257 units/L). Abdominal ultrasound revealed borderline hepatosplenomegaly. An ophthalmologic examination was normal.
On postoperative day 4 he developed left upper thigh swelling. An MRI showed rim‐enhancing juxta‐articular complex fluid collections surrounding the left femur with decreased marrow enhancement of the left proximal femur (Figure 1).

The limited rheumatologic evaluation is unrevealing; the ANA result is nondiagnostic and the ASO titer is normal for age. Laboratories generally report adult normal values for streptococcal antibodies regardless of the patient's age; children from ages 7 to 12 years are at their life peak frequency of group A streptococcal pharyngitis and typically have higher normal values of streptococcal antibodies, including ASO (up to about 480640 IU). The moderately elevated ferritin level is most likely an acute phase reactant and not high enough to suggest macrophage activation syndrome, which is unlikely with the normal AST, ALT, and LDH levels, the absence of significant splenomegaly, and the lack of cytopenias. Continued fever with progressive left upper thigh swelling point to osteomyelitis of the proximal femur, which may have initially ruptured into the hip and then infiltrated the femoral cortex and spread infection into the adjacent soft tissues. Surgical debridement is indicated.
The relative prevalence of methicillin‐sensitive S aureus (MSSA) and methicillin‐resistant S aureus vary widely with geography. MSSA strains are more likely to be highly toxigenic. The elaboration of 1 or more extracellular toxins could account for the patient's initial symptoms.
The patient was brought back to the operating room for drainage of the juxta‐articular fluid collections and a biopsy of his femur. The fluid collections were grossly purulent. His intraoperative cultures were positive for MSSA. The bone biopsy revealed necrotic tissue, acute inflammation, and bacterial colonies, consistent with acute osteomyelitis. Further testing of his S aureus isolate was positive for staphylococcal enterotoxin B. He completed a 4‐week course of oral clindamycin with subsequent normalization of his hip exam and inflammatory markers. At a follow‐up visit the patient was feeling better, but had developed skin peeling on the lateral aspects of his feet consistent with late sequelae of toxin‐mediated disease (Figure 2). Three months after discharge the patient had returned to his baseline activity level and remained asymptomatic.

COMMENTARY
The patient presented with a constellation of symptoms that was initially mistaken for incomplete KD until focal progression of his symptoms exposed an underlying femoral osteomyelitis with periarticular abscess formation. Bacterial cultures and subsequent toxin assay revealed an enterotoxin B‐producing strain of S aureus.
Certain staphylococcal strains secrete superantigens that may lead to the development of a systemic toxin‐mediated syndrome. Toxins elaborated by S aureus include toxic shock syndrome toxin‐1 (TSST‐1) and enterotoxins, which have been implicated in menstrual and nonmenstrual toxic shock syndromes.[1, 2] Enterotoxin B is a staphylococcal superantigen found in most strains of the USA400 clonal group, and has been frequently associated with skin and soft tissue infections.[3] Enterotoxin B production has been reported in nearly half of S aureus isolates from skin, soft tissue, and bone infections.[4]
Staphylococcal and streptococcal toxin‐mediated diseases can mimic vasculitis, systemic juvenile idiopathic arthritis, viral infections, and Stevens‐Johnson syndrome. Glossitis in toxin‐mediated syndromes manifests with a swollen, red tongue with overlying enlarged papillae, giving the appearance of a strawberry. Although pediatric providers often equate strawberry tongue, conjunctival injection, rash, and erythematous lips with KD, these findings are also seen in toxin‐mediated diseases, such as scarlet fever or staphylococcal toxic shock syndrome. Enterotoxin B mediated staphylococcal disease masquerading as KD has been reported in 2 cases: a 7‐month‐old boy with multifocal S aureus osteomyelitis and a 5‐year‐old boy with S aureus bacteremia. Both staphylococcal isolates produced enterotoxin B but were negative for other staphylococcus‐related toxins including TSST‐1.[5]
The Institute of Medicine (IOM) recently released its report Improving Diagnosis in Health Care, highlighting the under‐recognized quality and safety issue of diagnostic error.[6] The report uses the following broad and patient‐centered definition of diagnostic error: the failure to (a) establish an accurate and timely explanation of the patient's health problem(s) or (b) communicate that explanation to the patient. The IOM's conceptual model of diagnosis emphasizes the iterative nature of the diagnostic process, including the importance of generating a working diagnosis, gathering and incorporating new information in the reassessment of that diagnosis, and integrating treatment response into the formulation of the final diagnosis (Figure 3).

Even though the patient initially had several features consistent with KD, the increasing number of atypical features could have prompted the clinical team to reconsider their working diagnosis. The patient's age was atypical for KD, he had progressive knee and hip arthritis, and his fevers persisted after IVIG. An expanded differential should have included toxic shock syndrome; the resolution of conjunctival and mucosal injection and edema after IVIG may have been the result of antibodies in the IVIG preparation with neutralizing activity against superantigens. This antitoxin activity has established a role for IVIG in the management of staphylococcal toxic shock syndrome.[7] Ultimately, his imaging and surgical drainage revealed a focal staphylococcal toxin‐producing infectious source from which his fevers, rash, and mucosal and extremity changes emanated. This case reminds us that the more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should take it for another spin around the diagnostic wheel in search of a more suitable alternative.
KEY LEARNING POINTS
- Staphylococcal toxin‐mediated disease may mimic KD, with common features including strawberry tongue, oral and conjunctival injection, and skin desquamation.
- Improvement after treatment with IVIG is characteristic but not diagnostic of KD, and may be seen in toxin‐mediated disease.
- KD may present with arthralgia or arthritis, but severe joint abnormalities warrant consideration of infectious and other autoimmune conditions.
- The more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should gather, interpret, and integrate new information in search of a more suitable alternative.
Disclosure: Nothing to report.
A previously healthy 11‐year‐old boy presented to the emergency department after referral from his pediatrician for 1 week of fevers. Seven days prior to admission he developed a fever to 40.8C, vomiting, and mild left knee pain. The vomiting resolved within 2 days. Five days prior to admission he developed a pruritic, pinpoint rash over his abdomen that resolved within 24 hours. He also developed red, cracked lips, redness of his tongue, redness surrounding his eyes, and slight swelling of his hands. Three days prior to admission his pediatrician noted a 1‐cm anterior cervical lymph node. His fevers occurred throughout each of the prior 7 days without a discernible pattern, and his mild knee pain persisted at the time of presentation.
This preteen has had high fevers for 1 week associated with arthralgia, pruritic rash, emesis, and oral mucosal erythema. His rash, lip and tongue erythema, and swollen hands are classic features of Kawasaki disease (KD), but he lacks the other characteristic physical examination findings. The diagnosis of KD requires fever for at least 5 days accompanied by 4 of the following 5 signs: polymorphous rash, oral mucous membrane changes, peripheral extremity changes such as swelling or skin desquamation, bilateral bulbar conjunctival injection, and cervical lymphadenopathy >1.5 cm in diameter. Children meeting fewer than 4 of these criteria may have an incomplete form of KD.
Because most patients with KD (80%) are under 5 years old, alternative diagnoses such as autoimmune illnesses or a hypersensitivity reaction should be considered. Travel, medication, and animal exposure histories may reveal clues to an infectious or drug‐induced etiology of his fever. Immunization status should be assessed, as measles is also associated with fever, rash, and mucosal changes. Arthralgia or arthritis may occur in KD, but these findings suggest the need to entertain other possibilities, including bone or joint infection, infective endocarditis, inflammatory bowel disease, juvenile idiopathic arthritis (JIA), or systemic lupus erythematosus (SLE).
The child's only past medical history was an episode of croup as an infant. There was no family history of autoimmune diseases. He was not taking any medications and had no known allergies. His immunizations were up to date, including measles, mumps, rubella, and varicella. He lived with his parents and his dog. He swam in fresh water during a trip to Maine 2 months earlier. Neither he nor his family recalled a tick bite. He had no exposure to raw meat or unpasteurized dairy products.
The travel to New England raises the possibility of Lyme disease, although a 2‐month interval between exposure and a high, prolonged fever would be very unusual. Knee arthralgia or arthritis is common in children with late‐stage Lyme disease, but can also be seen in early‐disseminated disease. The prior description of the rash is not suggestive of erythema chronicum migrans, which is seen in early‐stage Lyme disease.
C‐reactive protein (CRP) was 189 mg/L (normal <6.3 mg/L). An echocardiogram was normal. Intravenous immunoglobulin (IVIG) was administered for presumed KD, with immediate improvement of the periorbital erythema, tongue redness, and hand swelling. He was discharged the next day on aspirin with cardiology clinic follow‐up.
Improvement after IVIG supports the diagnosis of KD. It is typical to discharge KD patients from the hospital when they have been afebrile for 24 hours or when the CRP level has declined by approximately 50%.
Over the next 48 hours he felt unwell with high‐grade fevers, continued left knee pain, and new left hip pain. He was readmitted to the hospital. His temperature was 39.4C, respiratory rate was 22 breaths per minute, heart rate was 122 beats per minute, blood pressure was 103/50 mm Hg, and oxygen saturation was 100% while breathing ambient air. He appeared mildly uncomfortable. His conjunctivae were normal. His lips were dry, red, and cracked, and his tongue was red with prominent papillae. His neck was supple without lymphadenopathy. His lungs were clear to auscultation. His heart exam was without murmurs. His abdomen was soft, and the liver and spleen were not enlarged. He had no swelling or erythema of his joints; however, he experienced pain with range of motion of his left knee, and tenderness and restricted range of motion of his left hip. His neurologic exam was normal. There were no rashes.
He has persistent fever, tachycardia, and tachypnea, now without features of KD except oral mucosal changes including prominent tongue papillae consistent with a strawberry tongue. Continued or recurrent fever may suggest persistent KD with ongoing inflammation or the need to search for an alternative diagnoses. An echocardiogram should be repeated, as the coronary artery abnormalities in KD can evolve rapidly, particularly when inflammation persists. Additional findings may include decreased left ventricular function, mitral regurgitation, or pericardial effusion. A second dose of IVIG is necessary to control fever and inflammation in about 15% of patients with KD, although in this case IVIG should be withheld pending further evaluation.
Arthralgia occurs commonly in KD, whereas frank arthritis is less typical. Polyarticular or oligoarticular arthritis involving small or large joints (especially knee or ankle) affects 5% to 10% of patients. The severity of findings in his left hip warrants consideration of septic arthritis with pain referred to the knee; pelvic or femoral osteomyelitis; psoas abscess; or pyomyositis. Following basic lab tests, imaging of the left hip region is indicated.
Laboratory evaluation revealed: white blood cell (WBC) count 10,000/L (absolute neutrophil count 8,460/L, absolute lymphocyte count 530/L), hemoglobin 10.6 g/dL, platelet count 208,000/L, serum sodium 130 mmol/L, serum potassium 3.3 mmol/L, serum urea nitrogen 11 mg/dL, serum creatinine 0.54 mg/dL, aspartate transaminase (AST) 26 U/L, alanine transaminase (ALT) 31 U/L, albumin 1.7 g/dL, erythrocyte sedimentation rate (ESR) > 100 mm/h, and CRP 263 mg/L. No blast cells were seen on peripheral blood smear.
Hypoalbuminemia and markedly elevated inflammatory markers indicate an inflammatory condition that has been active for more than a week. Assessing ESR after IVIG therapy is not useful because exogenous globulins increase the ESR; however, CRP is useful to monitor inflammation and remains elevated here.
Incomplete KD is still possible. Hyponatremia, hypoalbuminemia, and anemia are all features of persistent KD, and have been utilized in several clinical scoring systems in Japan to identify KD patients at increased risk for developing coronary complications. A neoplastic process cannot be excluded, but does not appear likely based on the acuity of his presentation and peripheral blood smear review.
Upon readmission he received a second dose of 2 g/kg IVIG. He remained on aspirin and continued to have fevers. A repeat echocardiogram was normal. He had worsening pain in his left knee and hip with difficulty straightening his left leg. Physical examination was notable for tenderness to palpation over his left hip joint, refusal to bear weight, and resistance to passive range of motion. On hospital day 2, an ultrasound of his left hip and knee revealed a complex left hip effusion and small left knee effusion.
KD becomes less likely in the presence of persistent fevers after IVIG and a repeatedly normal echocardiogram. Worsening left leg symptoms including impaired hip extension with a complex hip effusion suggests an infectious process in or adjacent to the left hip, such as septic arthritis, myositis, or osteomyelitis of the pelvis or proximal femur. A complex hip effusion is less likely to be present with arthritis related to JIA or SLE. The patient needs an emergent hip aspiration and possibly magnetic resonance imaging (MRI) to evaluate adjacent structures.
Arthrotomy and open drainage of his left hip revealed purulent fluid with a WBC count of 49,000/L with 89% neutrophils and 2% lymphocytes. Gram stain was negative. A left knee aspirate demonstrated straw‐colored synovial fluid (which was not sent for cell counts). Bacterial, fungal, and acid‐fast bacilli cultures were requested from hip and knee aspirates. Intravenous ceftriaxone and vancomycin were administered.
The most likely organism in pediatric pyogenic arthritis is Staphylococcus aureus, but there is a long list of other potential pathogens, including Streptococcus pyogenes (group A streptococcus) and Streptococcus pneumoniae. Most pediatric patients with acute pyogenic arthritis have synovial fluid WBC counts in excess of 75,000 to 100,000/L. The protracted course and the initial lack of hip symptoms raise the possibility of a primary osteomyelitis of the femur (particularly the intracapsular portion of the femoral neck or head) or of the acetabulum, with subsequent extension into the hip joint. Pyogenic myositis involving muscle groups adjacent to the hip would be unlikely to spread into the hip space, but can lead to synovial irritation, characterized by sterile joint fluid and WBC counts that fall short of the usual numbers seen in septic arthritis. The blood supply to the femoral head can become compromised with prolonged inflammation and increased intracapsular pressure, resulting in aseptic necrosis.
All cultures from his hip and knee aspirations were sterile. He continued to have daily fevers and persistent tachycardia while receiving intravenous ceftriaxone and vancomycin. Additional testing was notable for: antinuclear antibody (ANA) 1:80, anti‐streptolysin O (ASO) titer 344 IU (normal <150 IU), AST and ALT within normal limits, ferritin 568 ng/mL (normal <322 ng/mL), and lactate dehydrogenase (LDH) 212 units/L (normal <257 units/L). Abdominal ultrasound revealed borderline hepatosplenomegaly. An ophthalmologic examination was normal.
On postoperative day 4 he developed left upper thigh swelling. An MRI showed rim‐enhancing juxta‐articular complex fluid collections surrounding the left femur with decreased marrow enhancement of the left proximal femur (Figure 1).

The limited rheumatologic evaluation is unrevealing; the ANA result is nondiagnostic and the ASO titer is normal for age. Laboratories generally report adult normal values for streptococcal antibodies regardless of the patient's age; children from ages 7 to 12 years are at their life peak frequency of group A streptococcal pharyngitis and typically have higher normal values of streptococcal antibodies, including ASO (up to about 480640 IU). The moderately elevated ferritin level is most likely an acute phase reactant and not high enough to suggest macrophage activation syndrome, which is unlikely with the normal AST, ALT, and LDH levels, the absence of significant splenomegaly, and the lack of cytopenias. Continued fever with progressive left upper thigh swelling point to osteomyelitis of the proximal femur, which may have initially ruptured into the hip and then infiltrated the femoral cortex and spread infection into the adjacent soft tissues. Surgical debridement is indicated.
The relative prevalence of methicillin‐sensitive S aureus (MSSA) and methicillin‐resistant S aureus vary widely with geography. MSSA strains are more likely to be highly toxigenic. The elaboration of 1 or more extracellular toxins could account for the patient's initial symptoms.
The patient was brought back to the operating room for drainage of the juxta‐articular fluid collections and a biopsy of his femur. The fluid collections were grossly purulent. His intraoperative cultures were positive for MSSA. The bone biopsy revealed necrotic tissue, acute inflammation, and bacterial colonies, consistent with acute osteomyelitis. Further testing of his S aureus isolate was positive for staphylococcal enterotoxin B. He completed a 4‐week course of oral clindamycin with subsequent normalization of his hip exam and inflammatory markers. At a follow‐up visit the patient was feeling better, but had developed skin peeling on the lateral aspects of his feet consistent with late sequelae of toxin‐mediated disease (Figure 2). Three months after discharge the patient had returned to his baseline activity level and remained asymptomatic.

COMMENTARY
The patient presented with a constellation of symptoms that was initially mistaken for incomplete KD until focal progression of his symptoms exposed an underlying femoral osteomyelitis with periarticular abscess formation. Bacterial cultures and subsequent toxin assay revealed an enterotoxin B‐producing strain of S aureus.
Certain staphylococcal strains secrete superantigens that may lead to the development of a systemic toxin‐mediated syndrome. Toxins elaborated by S aureus include toxic shock syndrome toxin‐1 (TSST‐1) and enterotoxins, which have been implicated in menstrual and nonmenstrual toxic shock syndromes.[1, 2] Enterotoxin B is a staphylococcal superantigen found in most strains of the USA400 clonal group, and has been frequently associated with skin and soft tissue infections.[3] Enterotoxin B production has been reported in nearly half of S aureus isolates from skin, soft tissue, and bone infections.[4]
Staphylococcal and streptococcal toxin‐mediated diseases can mimic vasculitis, systemic juvenile idiopathic arthritis, viral infections, and Stevens‐Johnson syndrome. Glossitis in toxin‐mediated syndromes manifests with a swollen, red tongue with overlying enlarged papillae, giving the appearance of a strawberry. Although pediatric providers often equate strawberry tongue, conjunctival injection, rash, and erythematous lips with KD, these findings are also seen in toxin‐mediated diseases, such as scarlet fever or staphylococcal toxic shock syndrome. Enterotoxin B mediated staphylococcal disease masquerading as KD has been reported in 2 cases: a 7‐month‐old boy with multifocal S aureus osteomyelitis and a 5‐year‐old boy with S aureus bacteremia. Both staphylococcal isolates produced enterotoxin B but were negative for other staphylococcus‐related toxins including TSST‐1.[5]
The Institute of Medicine (IOM) recently released its report Improving Diagnosis in Health Care, highlighting the under‐recognized quality and safety issue of diagnostic error.[6] The report uses the following broad and patient‐centered definition of diagnostic error: the failure to (a) establish an accurate and timely explanation of the patient's health problem(s) or (b) communicate that explanation to the patient. The IOM's conceptual model of diagnosis emphasizes the iterative nature of the diagnostic process, including the importance of generating a working diagnosis, gathering and incorporating new information in the reassessment of that diagnosis, and integrating treatment response into the formulation of the final diagnosis (Figure 3).

Even though the patient initially had several features consistent with KD, the increasing number of atypical features could have prompted the clinical team to reconsider their working diagnosis. The patient's age was atypical for KD, he had progressive knee and hip arthritis, and his fevers persisted after IVIG. An expanded differential should have included toxic shock syndrome; the resolution of conjunctival and mucosal injection and edema after IVIG may have been the result of antibodies in the IVIG preparation with neutralizing activity against superantigens. This antitoxin activity has established a role for IVIG in the management of staphylococcal toxic shock syndrome.[7] Ultimately, his imaging and surgical drainage revealed a focal staphylococcal toxin‐producing infectious source from which his fevers, rash, and mucosal and extremity changes emanated. This case reminds us that the more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should take it for another spin around the diagnostic wheel in search of a more suitable alternative.
KEY LEARNING POINTS
- Staphylococcal toxin‐mediated disease may mimic KD, with common features including strawberry tongue, oral and conjunctival injection, and skin desquamation.
- Improvement after treatment with IVIG is characteristic but not diagnostic of KD, and may be seen in toxin‐mediated disease.
- KD may present with arthralgia or arthritis, but severe joint abnormalities warrant consideration of infectious and other autoimmune conditions.
- The more atypical a working diagnosis iseither in its presentation or treatment responsethe more readily clinicians should gather, interpret, and integrate new information in search of a more suitable alternative.
Disclosure: Nothing to report.
- . Staphylococcal enterotoxin B and toxic shock syndrome toxin‐1 are significantly associated with non‐menstrual TSS. Lancet. 1986;1:1149–1150.
- , , , , . Toxic shock syndrome caused by a strain of staphylococcus aureus that produces enterotoxin C but not toxic shock syndrome toxin‐1. Am J Dis Child. 1989;143 (7):848–849.
- , , , , , . Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS One. 2012;7(7):e41157.
- , , , et al. Variability of antibiotic susceptibility and toxin production of Staphylococcal aureus stains isolated from skin, soft tissue, and bone related infections. BMC Microbiol. 2013;13:188.
- , , , , . Kawasaki syndrome‐like illness associated with infection caused by enterotoxin B‐secreting Staphylococcus aureus. Clin Microbiol Rev. 2013;26:422–447.
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015.
- , , , et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clin Infect Dis. 2003;37(3):333–340.
- . Staphylococcal enterotoxin B and toxic shock syndrome toxin‐1 are significantly associated with non‐menstrual TSS. Lancet. 1986;1:1149–1150.
- , , , , . Toxic shock syndrome caused by a strain of staphylococcus aureus that produces enterotoxin C but not toxic shock syndrome toxin‐1. Am J Dis Child. 1989;143 (7):848–849.
- , , , , , . Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS One. 2012;7(7):e41157.
- , , , et al. Variability of antibiotic susceptibility and toxin production of Staphylococcal aureus stains isolated from skin, soft tissue, and bone related infections. BMC Microbiol. 2013;13:188.
- , , , , . Kawasaki syndrome‐like illness associated with infection caused by enterotoxin B‐secreting Staphylococcus aureus. Clin Microbiol Rev. 2013;26:422–447.
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015.
- , , , et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clin Infect Dis. 2003;37(3):333–340.