User login
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
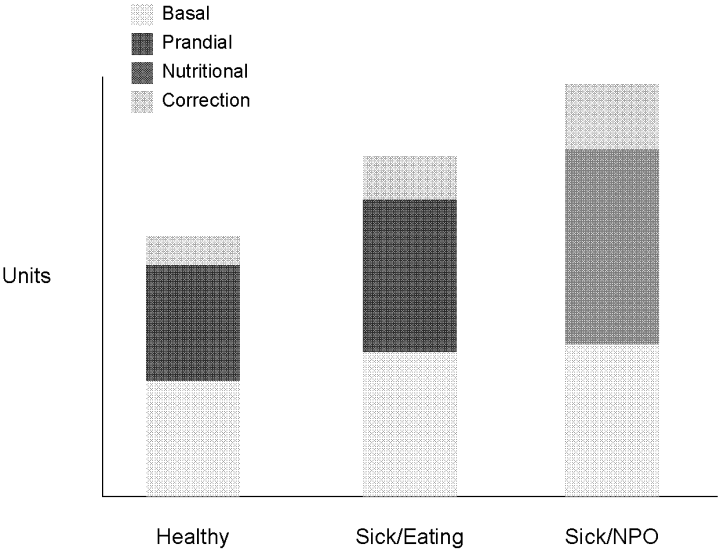
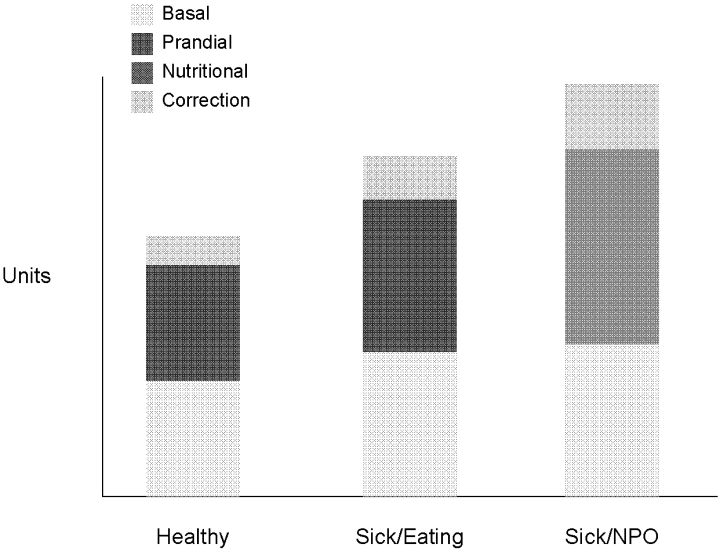
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.
| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.

| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.

| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.