User login
In co-management, do what’s best for the patient in a timely fashion
Deferring management of a postop complication to the surgery team resulted in treatment delay with a serious adverse outcome.
History:
RR is a 54 year-old man with a medical history of hypertension, hyperlipidemia, obesity, and chronic left knee pain from osteoarthritis. He was admitted to the hospital and underwent an elective left total knee replacement with monitored anesthesia care, combined with a left femoral nerve block. There were no intraoperative complications. When RR awoke in the recovery unit, he was in excruciating pain. He received another femoral nerve block and was sent to the regular nursing floor around 4 p.m. By early evening, the pain in his left leg remained poorly controlled and was consistently rated as 10/10. In addition, RR’s heart rates were elevated (130-140 bpm). The orthopedic surgeon was notified of the uncontrolled pain and elevated heart rates and he requested a hospitalist consult.
Dr. Hospitalist saw RR sometime before 9 p.m. that evening. RR was somewhat sedated by opiate analgesics, and his wife was at the bedside. During the interview, she related that her husband had been taking nightly benzodiazepines for sleep for several months leading up to the surgery. Dr. Hospitalist did not examine RR’s left foot and leg, and he documented in his consult that he was deferring left leg issues such as bleeding and swelling to the orthopedic surgery team. Dr. Hospitalist’s impression was that RR had sinus tachycardia, possibly because of the benzodiazepine withdrawal. Fluids were ordered along with low-dose benzodiazepines.
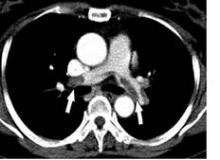
Throughout the night, RR awakened and complained of severe pain. The evening nurse charted that RR was having difficulty moving the toes on his left foot and that the pulses in his foot were barely palpable. By the early morning, RR’s pulses were no longer palpable but could still be detected by Doppler. Examination by the surgical team the following morning documented that RR had decreased sensation in his left lower extremity as well. An ultrasound of the left leg was ordered and revealed a large left popliteal pseudoaneurysm with complete occlusion of the left popliteal, tibial, and peroneal arteries below the knee. The patient went to the operating room three times over the next 4 days in an attempt to revascularize the leg. Unfortunately, RR ultimately had an above-the-knee amputation (AKA) performed 9 days after his elective total knee replacement.
Complaint:
RR sought a “quality of life”–enhancing procedure for his chronic left knee pain. What he ended up with was an AKA and a significant decrease in his overall quality of life. RR was angry that his postoperative pain, which was out of proportion to what should have been expected for this type of surgery, was essentially ignored until it was too late. He blamed the surgeon and the hospitalist for failing to diagnose his condition while his leg was still salvageable.
Scientific principles:
Complications during and after total knee replacement are generally uncommon and can often be prevented with meticulous surgical technique and with attentive postoperative management. Vascular injuries in total knee arthroplasty are exceedingly rare, but careful examination of the limbs is necessary to detect signs of acute limb ischemia. The six P’s of acute ischemia include paresthesia, pain, pallor, pulselessness, poikilothermia, and paralysis. A diagnosis of acute lower extremity ischemia can generally be made based upon the history and physical examination. Once the diagnosis of acute arterial occlusion has been made, anticoagulation should be initiated. Subsequent treatment varies depending upon the classification of acute ischemia. The initial options include catheter-directed thrombolytic therapy with or without percutaneous intervention or surgery.
Complaint rebuttal and discussion:
Dr. Hospitalist defended himself by limiting his scope of responsibility. He essentially said this was a surgical complication, and it was therefore the surgical team’s responsibility to make the diagnosis. Defense experts were quick to affirm that Dr. Hospitalist was consulted for a specific issue – postoperative tachycardia – and that he performed a focused history and physical examination to address that issue. Plaintiff experts cited the Society of Hospital Medicine Hospitalist Orthopedic Co-Management Implementation Guide, which outlines that co-management is the “shared responsibility, authority, and accountability for the care of a hospitalized patient.” The Guide further states: “Inevitably, there will be circumstances where either of the co-managing services could manage a specific problem, or where it is unclear which service would be best equipped to manage a specific problem. These situations can be best managed by following two basic principles: 1) Do what is best for the patient in a timely fashion and do not assume that a problem is being handled by the other service; and 2) communicate frequently and directly with the other service.” Plaintiff experts argued that Dr. Hospitalist failed to follow both of these principles.
Conclusion:
Hospitalists are frequently co-managers of surgical patients, and thus they are in part responsible for evaluating diagnosing, and treating both medical and surgical complications. Once again, it is vital that hospitalists delineate responsibilities explicitly through direct communication and then memorialize such discussions in the medical record. In this case, the chart consultation deferred examination of the operative leg to a surgical team that claimed they were “unaware” of any issues. This case was settled on behalf of the patient for an undisclosed amount.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Deferring management of a postop complication to the surgery team resulted in treatment delay with a serious adverse outcome.
History:
RR is a 54 year-old man with a medical history of hypertension, hyperlipidemia, obesity, and chronic left knee pain from osteoarthritis. He was admitted to the hospital and underwent an elective left total knee replacement with monitored anesthesia care, combined with a left femoral nerve block. There were no intraoperative complications. When RR awoke in the recovery unit, he was in excruciating pain. He received another femoral nerve block and was sent to the regular nursing floor around 4 p.m. By early evening, the pain in his left leg remained poorly controlled and was consistently rated as 10/10. In addition, RR’s heart rates were elevated (130-140 bpm). The orthopedic surgeon was notified of the uncontrolled pain and elevated heart rates and he requested a hospitalist consult.
Dr. Hospitalist saw RR sometime before 9 p.m. that evening. RR was somewhat sedated by opiate analgesics, and his wife was at the bedside. During the interview, she related that her husband had been taking nightly benzodiazepines for sleep for several months leading up to the surgery. Dr. Hospitalist did not examine RR’s left foot and leg, and he documented in his consult that he was deferring left leg issues such as bleeding and swelling to the orthopedic surgery team. Dr. Hospitalist’s impression was that RR had sinus tachycardia, possibly because of the benzodiazepine withdrawal. Fluids were ordered along with low-dose benzodiazepines.
Throughout the night, RR awakened and complained of severe pain. The evening nurse charted that RR was having difficulty moving the toes on his left foot and that the pulses in his foot were barely palpable. By the early morning, RR’s pulses were no longer palpable but could still be detected by Doppler. Examination by the surgical team the following morning documented that RR had decreased sensation in his left lower extremity as well. An ultrasound of the left leg was ordered and revealed a large left popliteal pseudoaneurysm with complete occlusion of the left popliteal, tibial, and peroneal arteries below the knee. The patient went to the operating room three times over the next 4 days in an attempt to revascularize the leg. Unfortunately, RR ultimately had an above-the-knee amputation (AKA) performed 9 days after his elective total knee replacement.
Complaint:
RR sought a “quality of life”–enhancing procedure for his chronic left knee pain. What he ended up with was an AKA and a significant decrease in his overall quality of life. RR was angry that his postoperative pain, which was out of proportion to what should have been expected for this type of surgery, was essentially ignored until it was too late. He blamed the surgeon and the hospitalist for failing to diagnose his condition while his leg was still salvageable.
Scientific principles:
Complications during and after total knee replacement are generally uncommon and can often be prevented with meticulous surgical technique and with attentive postoperative management. Vascular injuries in total knee arthroplasty are exceedingly rare, but careful examination of the limbs is necessary to detect signs of acute limb ischemia. The six P’s of acute ischemia include paresthesia, pain, pallor, pulselessness, poikilothermia, and paralysis. A diagnosis of acute lower extremity ischemia can generally be made based upon the history and physical examination. Once the diagnosis of acute arterial occlusion has been made, anticoagulation should be initiated. Subsequent treatment varies depending upon the classification of acute ischemia. The initial options include catheter-directed thrombolytic therapy with or without percutaneous intervention or surgery.
Complaint rebuttal and discussion:
Dr. Hospitalist defended himself by limiting his scope of responsibility. He essentially said this was a surgical complication, and it was therefore the surgical team’s responsibility to make the diagnosis. Defense experts were quick to affirm that Dr. Hospitalist was consulted for a specific issue – postoperative tachycardia – and that he performed a focused history and physical examination to address that issue. Plaintiff experts cited the Society of Hospital Medicine Hospitalist Orthopedic Co-Management Implementation Guide, which outlines that co-management is the “shared responsibility, authority, and accountability for the care of a hospitalized patient.” The Guide further states: “Inevitably, there will be circumstances where either of the co-managing services could manage a specific problem, or where it is unclear which service would be best equipped to manage a specific problem. These situations can be best managed by following two basic principles: 1) Do what is best for the patient in a timely fashion and do not assume that a problem is being handled by the other service; and 2) communicate frequently and directly with the other service.” Plaintiff experts argued that Dr. Hospitalist failed to follow both of these principles.
Conclusion:
Hospitalists are frequently co-managers of surgical patients, and thus they are in part responsible for evaluating diagnosing, and treating both medical and surgical complications. Once again, it is vital that hospitalists delineate responsibilities explicitly through direct communication and then memorialize such discussions in the medical record. In this case, the chart consultation deferred examination of the operative leg to a surgical team that claimed they were “unaware” of any issues. This case was settled on behalf of the patient for an undisclosed amount.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Deferring management of a postop complication to the surgery team resulted in treatment delay with a serious adverse outcome.
History:
RR is a 54 year-old man with a medical history of hypertension, hyperlipidemia, obesity, and chronic left knee pain from osteoarthritis. He was admitted to the hospital and underwent an elective left total knee replacement with monitored anesthesia care, combined with a left femoral nerve block. There were no intraoperative complications. When RR awoke in the recovery unit, he was in excruciating pain. He received another femoral nerve block and was sent to the regular nursing floor around 4 p.m. By early evening, the pain in his left leg remained poorly controlled and was consistently rated as 10/10. In addition, RR’s heart rates were elevated (130-140 bpm). The orthopedic surgeon was notified of the uncontrolled pain and elevated heart rates and he requested a hospitalist consult.
Dr. Hospitalist saw RR sometime before 9 p.m. that evening. RR was somewhat sedated by opiate analgesics, and his wife was at the bedside. During the interview, she related that her husband had been taking nightly benzodiazepines for sleep for several months leading up to the surgery. Dr. Hospitalist did not examine RR’s left foot and leg, and he documented in his consult that he was deferring left leg issues such as bleeding and swelling to the orthopedic surgery team. Dr. Hospitalist’s impression was that RR had sinus tachycardia, possibly because of the benzodiazepine withdrawal. Fluids were ordered along with low-dose benzodiazepines.
Throughout the night, RR awakened and complained of severe pain. The evening nurse charted that RR was having difficulty moving the toes on his left foot and that the pulses in his foot were barely palpable. By the early morning, RR’s pulses were no longer palpable but could still be detected by Doppler. Examination by the surgical team the following morning documented that RR had decreased sensation in his left lower extremity as well. An ultrasound of the left leg was ordered and revealed a large left popliteal pseudoaneurysm with complete occlusion of the left popliteal, tibial, and peroneal arteries below the knee. The patient went to the operating room three times over the next 4 days in an attempt to revascularize the leg. Unfortunately, RR ultimately had an above-the-knee amputation (AKA) performed 9 days after his elective total knee replacement.
Complaint:
RR sought a “quality of life”–enhancing procedure for his chronic left knee pain. What he ended up with was an AKA and a significant decrease in his overall quality of life. RR was angry that his postoperative pain, which was out of proportion to what should have been expected for this type of surgery, was essentially ignored until it was too late. He blamed the surgeon and the hospitalist for failing to diagnose his condition while his leg was still salvageable.
Scientific principles:
Complications during and after total knee replacement are generally uncommon and can often be prevented with meticulous surgical technique and with attentive postoperative management. Vascular injuries in total knee arthroplasty are exceedingly rare, but careful examination of the limbs is necessary to detect signs of acute limb ischemia. The six P’s of acute ischemia include paresthesia, pain, pallor, pulselessness, poikilothermia, and paralysis. A diagnosis of acute lower extremity ischemia can generally be made based upon the history and physical examination. Once the diagnosis of acute arterial occlusion has been made, anticoagulation should be initiated. Subsequent treatment varies depending upon the classification of acute ischemia. The initial options include catheter-directed thrombolytic therapy with or without percutaneous intervention or surgery.
Complaint rebuttal and discussion:
Dr. Hospitalist defended himself by limiting his scope of responsibility. He essentially said this was a surgical complication, and it was therefore the surgical team’s responsibility to make the diagnosis. Defense experts were quick to affirm that Dr. Hospitalist was consulted for a specific issue – postoperative tachycardia – and that he performed a focused history and physical examination to address that issue. Plaintiff experts cited the Society of Hospital Medicine Hospitalist Orthopedic Co-Management Implementation Guide, which outlines that co-management is the “shared responsibility, authority, and accountability for the care of a hospitalized patient.” The Guide further states: “Inevitably, there will be circumstances where either of the co-managing services could manage a specific problem, or where it is unclear which service would be best equipped to manage a specific problem. These situations can be best managed by following two basic principles: 1) Do what is best for the patient in a timely fashion and do not assume that a problem is being handled by the other service; and 2) communicate frequently and directly with the other service.” Plaintiff experts argued that Dr. Hospitalist failed to follow both of these principles.
Conclusion:
Hospitalists are frequently co-managers of surgical patients, and thus they are in part responsible for evaluating diagnosing, and treating both medical and surgical complications. Once again, it is vital that hospitalists delineate responsibilities explicitly through direct communication and then memorialize such discussions in the medical record. In this case, the chart consultation deferred examination of the operative leg to a surgical team that claimed they were “unaware” of any issues. This case was settled on behalf of the patient for an undisclosed amount.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
For PE victim, would Wells’ have been enough?
Medicolegal review has the opportunity to become the morbidity and mortality conference of the modern era. The more we share in our collective failures, the less likely we are to repeat those same mistakes.
CR was a 69-year-old man who presented to the hospital for an elective total knee revision. He had a past medical history of obesity, hypertension, and chronic obstructive pulmonary disease (COPD).
Two weeks prior to the surgery, he received preoperative clearance from his primary care physician. CR had a morning surgery that went well and was without complication. He was transferred to the postanesthesia care unit in good condition with normal oxygen saturations on 2L of oxygen by nasal cannula. His postoperative orders included a routine hospitalist consultation for “medical management,” along with orders for daily low-molecular weight heparin for deep vein thrombosis (DVT) prophylaxis. CR arrived to the orthopedic floor later that afternoon. He did well overnight, and the next morning, he began working with physical therapy. After doing some exercises in the bed, CR had his oxygen removed so that he could ambulate. While up with the physical therapist, CR reported feeling “dizzy.” A chair was brought up behind him so that he could sit down. His oxygen saturation was 88%. CR was placed back on 2L of oxygen, but as he transferred from the chair to the bed his oxygen saturation fell further to 81%. CR had his oxygen increased to 3L and over the next half-hour, his oxygen saturation came up and stayed above 92%.
Dr. Hospitalist met CR about an hour after he worked with physical therapy. CR was without complaints at the time of the evaluation and had good oxygen saturations on 3L of oxygen. Dr. Hospitalist documented that CR denied chest pain, cough, or sputum production. On auscultation, CR had a scattered wheeze. Dr. Hospitalist dictated that his differential diagnosis included pulmonary embolism (PE). He ordered bronchodilator aerosols, a chest radiograph, a troponin and a brain natriuretic peptide (BNP) level. The chest radiograph was performed that afternoon and interpreted as “no acute process,” with no evidence for atelectasis.
Overnight, CR remained on oxygen via nasal cannula. The following morning his nurse noted that CR had bilateral edema in his legs. The labs ordered by Dr. Hospitalist the previous day were now in the chart – the troponin was 0.07 ng/mL (normal < 0.04 ng/ml) and the BNP was 205 pg/mL.
At 11 a.m., CR was again seen by physical therapy. While ambulating in his room, CR began to feel “dizzy” despite the use of oxygen, and he passed out falling to his knees. The therapist and several nurses got him back to a chair and increased his oxygen. He spontaneously regained consciousness, but within a few minutes CR passed out a second time and lost his pulse. Dr. Hospitalist and the surgeon responded to the code. He was unable to be resuscitated and was pronounced dead. An autopsy was performed and determined the cause of death to be massive saddle pulmonary embolism (PE).
Complaint:
A complaint was filed against the hospital, the surgeon, and Dr. Hospitalist for failure to prevent DVT, failure to diagnose PE, and failure to treat PE. The complaint alleged that had the standard of care been followed, CR would not have died postoperatively and would otherwise have had a normal life expectancy.
Scientific principles:
Orthopedic surgery patients are known to be at high risk for venous thromboembolism. In the absence of prophylaxis, postoperative PE is common and often a fatal disease. Nonetheless, even without prophylaxis, mortality from PE can be reduced by prompt diagnosis and therapy. Unfortunately, the clinical presentation of PE is variable and nonspecific; thus, diagnostic testing is necessary before confirming or excluding the diagnosis of PE. The diagnostic approach includes algorithms designed to efficiently diagnose PE while simultaneously avoiding unnecessary testing and minimizing the risk of missing clinically important cases. While there is consensus regarding the need for algorithms, there is no agreed-upon best approach.
Complaint rebuttal and discussion:
The defense responded that the first item in the complaint was baseless as the surgeon ordered both mechanical and low-molecular weight heparin prophylaxis for DVT. The plaintiff experts agreed and the surgeon was dismissed from the case prior to trial. The focus of the case was now on Dr. Hospitalist and his failure to diagnose and treat PE.
Dr. Hospitalist defended himself by arguing that CR suffered a sudden fatal PE on the day of his death despite appropriate prophylaxis and that the “dizziness” the prior day was unrelated. The defense explained that the day prior to his death, CR was simply orthostatic from his postanesthesia state, combined with opiate analgesics, and any hypoxia was from CR’s preexisting COPD.
Plaintiff experts replied that CR had virtually no symptoms for a COPD exacerbation (i.e., no cough, no sputum production), and there was no explanation for the elevated troponin other than PE. Plaintiff experts further alleged that Dr. Hospitalist failed to incorporate an algorithm, such as the modified Wells’ Criteria, into his diagnostic approach for PE. Had he done so, Dr. Hospitalist would have recognized that CR had a high enough clinical probability for PE to warrant empiric treatment and confirmatory testing. The defense responded that the use of the modified Wells’ Criteria was nothing but an arcane “academic” exercise that did not match real clinical practice.
Conclusion:
Acute pulmonary embolism is a well-known postoperative pulmonary complication. The diagnosis must be considered in any surgical patient that has postoperative shortness of breath or unexplained hypoxia. The importance of using an algorithm to determine the need for testing and treatment cannot be understated. In this case, the PE diagnosis was considered but no algorithm was used. The jury in this case deliberated for more than a day, but ultimately returned a full defense verdict.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Medicolegal review has the opportunity to become the morbidity and mortality conference of the modern era. The more we share in our collective failures, the less likely we are to repeat those same mistakes.
CR was a 69-year-old man who presented to the hospital for an elective total knee revision. He had a past medical history of obesity, hypertension, and chronic obstructive pulmonary disease (COPD).
Two weeks prior to the surgery, he received preoperative clearance from his primary care physician. CR had a morning surgery that went well and was without complication. He was transferred to the postanesthesia care unit in good condition with normal oxygen saturations on 2L of oxygen by nasal cannula. His postoperative orders included a routine hospitalist consultation for “medical management,” along with orders for daily low-molecular weight heparin for deep vein thrombosis (DVT) prophylaxis. CR arrived to the orthopedic floor later that afternoon. He did well overnight, and the next morning, he began working with physical therapy. After doing some exercises in the bed, CR had his oxygen removed so that he could ambulate. While up with the physical therapist, CR reported feeling “dizzy.” A chair was brought up behind him so that he could sit down. His oxygen saturation was 88%. CR was placed back on 2L of oxygen, but as he transferred from the chair to the bed his oxygen saturation fell further to 81%. CR had his oxygen increased to 3L and over the next half-hour, his oxygen saturation came up and stayed above 92%.
Dr. Hospitalist met CR about an hour after he worked with physical therapy. CR was without complaints at the time of the evaluation and had good oxygen saturations on 3L of oxygen. Dr. Hospitalist documented that CR denied chest pain, cough, or sputum production. On auscultation, CR had a scattered wheeze. Dr. Hospitalist dictated that his differential diagnosis included pulmonary embolism (PE). He ordered bronchodilator aerosols, a chest radiograph, a troponin and a brain natriuretic peptide (BNP) level. The chest radiograph was performed that afternoon and interpreted as “no acute process,” with no evidence for atelectasis.
Overnight, CR remained on oxygen via nasal cannula. The following morning his nurse noted that CR had bilateral edema in his legs. The labs ordered by Dr. Hospitalist the previous day were now in the chart – the troponin was 0.07 ng/mL (normal < 0.04 ng/ml) and the BNP was 205 pg/mL.
At 11 a.m., CR was again seen by physical therapy. While ambulating in his room, CR began to feel “dizzy” despite the use of oxygen, and he passed out falling to his knees. The therapist and several nurses got him back to a chair and increased his oxygen. He spontaneously regained consciousness, but within a few minutes CR passed out a second time and lost his pulse. Dr. Hospitalist and the surgeon responded to the code. He was unable to be resuscitated and was pronounced dead. An autopsy was performed and determined the cause of death to be massive saddle pulmonary embolism (PE).
Complaint:
A complaint was filed against the hospital, the surgeon, and Dr. Hospitalist for failure to prevent DVT, failure to diagnose PE, and failure to treat PE. The complaint alleged that had the standard of care been followed, CR would not have died postoperatively and would otherwise have had a normal life expectancy.
Scientific principles:
Orthopedic surgery patients are known to be at high risk for venous thromboembolism. In the absence of prophylaxis, postoperative PE is common and often a fatal disease. Nonetheless, even without prophylaxis, mortality from PE can be reduced by prompt diagnosis and therapy. Unfortunately, the clinical presentation of PE is variable and nonspecific; thus, diagnostic testing is necessary before confirming or excluding the diagnosis of PE. The diagnostic approach includes algorithms designed to efficiently diagnose PE while simultaneously avoiding unnecessary testing and minimizing the risk of missing clinically important cases. While there is consensus regarding the need for algorithms, there is no agreed-upon best approach.
Complaint rebuttal and discussion:
The defense responded that the first item in the complaint was baseless as the surgeon ordered both mechanical and low-molecular weight heparin prophylaxis for DVT. The plaintiff experts agreed and the surgeon was dismissed from the case prior to trial. The focus of the case was now on Dr. Hospitalist and his failure to diagnose and treat PE.
Dr. Hospitalist defended himself by arguing that CR suffered a sudden fatal PE on the day of his death despite appropriate prophylaxis and that the “dizziness” the prior day was unrelated. The defense explained that the day prior to his death, CR was simply orthostatic from his postanesthesia state, combined with opiate analgesics, and any hypoxia was from CR’s preexisting COPD.
Plaintiff experts replied that CR had virtually no symptoms for a COPD exacerbation (i.e., no cough, no sputum production), and there was no explanation for the elevated troponin other than PE. Plaintiff experts further alleged that Dr. Hospitalist failed to incorporate an algorithm, such as the modified Wells’ Criteria, into his diagnostic approach for PE. Had he done so, Dr. Hospitalist would have recognized that CR had a high enough clinical probability for PE to warrant empiric treatment and confirmatory testing. The defense responded that the use of the modified Wells’ Criteria was nothing but an arcane “academic” exercise that did not match real clinical practice.
Conclusion:
Acute pulmonary embolism is a well-known postoperative pulmonary complication. The diagnosis must be considered in any surgical patient that has postoperative shortness of breath or unexplained hypoxia. The importance of using an algorithm to determine the need for testing and treatment cannot be understated. In this case, the PE diagnosis was considered but no algorithm was used. The jury in this case deliberated for more than a day, but ultimately returned a full defense verdict.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Medicolegal review has the opportunity to become the morbidity and mortality conference of the modern era. The more we share in our collective failures, the less likely we are to repeat those same mistakes.
CR was a 69-year-old man who presented to the hospital for an elective total knee revision. He had a past medical history of obesity, hypertension, and chronic obstructive pulmonary disease (COPD).
Two weeks prior to the surgery, he received preoperative clearance from his primary care physician. CR had a morning surgery that went well and was without complication. He was transferred to the postanesthesia care unit in good condition with normal oxygen saturations on 2L of oxygen by nasal cannula. His postoperative orders included a routine hospitalist consultation for “medical management,” along with orders for daily low-molecular weight heparin for deep vein thrombosis (DVT) prophylaxis. CR arrived to the orthopedic floor later that afternoon. He did well overnight, and the next morning, he began working with physical therapy. After doing some exercises in the bed, CR had his oxygen removed so that he could ambulate. While up with the physical therapist, CR reported feeling “dizzy.” A chair was brought up behind him so that he could sit down. His oxygen saturation was 88%. CR was placed back on 2L of oxygen, but as he transferred from the chair to the bed his oxygen saturation fell further to 81%. CR had his oxygen increased to 3L and over the next half-hour, his oxygen saturation came up and stayed above 92%.
Dr. Hospitalist met CR about an hour after he worked with physical therapy. CR was without complaints at the time of the evaluation and had good oxygen saturations on 3L of oxygen. Dr. Hospitalist documented that CR denied chest pain, cough, or sputum production. On auscultation, CR had a scattered wheeze. Dr. Hospitalist dictated that his differential diagnosis included pulmonary embolism (PE). He ordered bronchodilator aerosols, a chest radiograph, a troponin and a brain natriuretic peptide (BNP) level. The chest radiograph was performed that afternoon and interpreted as “no acute process,” with no evidence for atelectasis.
Overnight, CR remained on oxygen via nasal cannula. The following morning his nurse noted that CR had bilateral edema in his legs. The labs ordered by Dr. Hospitalist the previous day were now in the chart – the troponin was 0.07 ng/mL (normal < 0.04 ng/ml) and the BNP was 205 pg/mL.
At 11 a.m., CR was again seen by physical therapy. While ambulating in his room, CR began to feel “dizzy” despite the use of oxygen, and he passed out falling to his knees. The therapist and several nurses got him back to a chair and increased his oxygen. He spontaneously regained consciousness, but within a few minutes CR passed out a second time and lost his pulse. Dr. Hospitalist and the surgeon responded to the code. He was unable to be resuscitated and was pronounced dead. An autopsy was performed and determined the cause of death to be massive saddle pulmonary embolism (PE).
Complaint:
A complaint was filed against the hospital, the surgeon, and Dr. Hospitalist for failure to prevent DVT, failure to diagnose PE, and failure to treat PE. The complaint alleged that had the standard of care been followed, CR would not have died postoperatively and would otherwise have had a normal life expectancy.
Scientific principles:
Orthopedic surgery patients are known to be at high risk for venous thromboembolism. In the absence of prophylaxis, postoperative PE is common and often a fatal disease. Nonetheless, even without prophylaxis, mortality from PE can be reduced by prompt diagnosis and therapy. Unfortunately, the clinical presentation of PE is variable and nonspecific; thus, diagnostic testing is necessary before confirming or excluding the diagnosis of PE. The diagnostic approach includes algorithms designed to efficiently diagnose PE while simultaneously avoiding unnecessary testing and minimizing the risk of missing clinically important cases. While there is consensus regarding the need for algorithms, there is no agreed-upon best approach.
Complaint rebuttal and discussion:
The defense responded that the first item in the complaint was baseless as the surgeon ordered both mechanical and low-molecular weight heparin prophylaxis for DVT. The plaintiff experts agreed and the surgeon was dismissed from the case prior to trial. The focus of the case was now on Dr. Hospitalist and his failure to diagnose and treat PE.
Dr. Hospitalist defended himself by arguing that CR suffered a sudden fatal PE on the day of his death despite appropriate prophylaxis and that the “dizziness” the prior day was unrelated. The defense explained that the day prior to his death, CR was simply orthostatic from his postanesthesia state, combined with opiate analgesics, and any hypoxia was from CR’s preexisting COPD.
Plaintiff experts replied that CR had virtually no symptoms for a COPD exacerbation (i.e., no cough, no sputum production), and there was no explanation for the elevated troponin other than PE. Plaintiff experts further alleged that Dr. Hospitalist failed to incorporate an algorithm, such as the modified Wells’ Criteria, into his diagnostic approach for PE. Had he done so, Dr. Hospitalist would have recognized that CR had a high enough clinical probability for PE to warrant empiric treatment and confirmatory testing. The defense responded that the use of the modified Wells’ Criteria was nothing but an arcane “academic” exercise that did not match real clinical practice.
Conclusion:
Acute pulmonary embolism is a well-known postoperative pulmonary complication. The diagnosis must be considered in any surgical patient that has postoperative shortness of breath or unexplained hypoxia. The importance of using an algorithm to determine the need for testing and treatment cannot be understated. In this case, the PE diagnosis was considered but no algorithm was used. The jury in this case deliberated for more than a day, but ultimately returned a full defense verdict.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Death by discontinuity of care
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
Managing diabetes in hemodialysis patients: Observations and recommendations
Although diabetes is the most common cause of end-stage renal disease (ESRD) worldwide, accounting for 44.2% of ESRD patients in the US Renal Data System in 2005,1 data are scarce on how diabetes should best be treated in patients in ESRD.
We do know that blood glucose levels need to be well controlled in these patients. Several observational studies and one nonrandomized interventional study2–10 showed that higher levels of hemoglobin A1c were associated with higher death rates in patients with diabetes and chronic kidney disease after adjusting for markers of inflammation and malnutrition.
However, ESRD significantly alters glycemic control, the results of hemoglobin A1c testing, and the excretion of antidiabetic medications. The various and opposing effects of ESRD and dialysis can make blood glucose levels fluctuate widely, placing patients at risk of hypoglycemia—and presenting a challenge for nephrologists and internists.
In this review, we summarize the available evidence and make practical recommendations for managing diabetes in patients on hemodialysis.
GLUCOSE LEVELS MAY FLUCTUATE WIDELY
In ESRD, both uremia and dialysis can complicate glycemic control by affecting the secretion, clearance, and peripheral tissue sensitivity of insulin.
Several factors, including uremic toxins, may increase insulin resistance in ESRD, leading to a blunted ability to suppress hepatic gluconeogenesis and regulate peripheral glucose utilization. In type 2 diabetes without kidney disease, insulin resistance leads to increased insulin secretion. This does not occur in ESRD because of concomitant metabolic acidosis, deficiency of 1,25 dihydroxyvitamin D, and secondary hyperparathyroidism.11,12 Hemodialysis further alters insulin secretion, clearance, and resistance as the result of periodic improvement in uremia, acidosis, and phosphate handling.
The dextrose concentration in the dialysate can also affect glucose control. In general, dialysates with lower dextrose concentrations are used and may be associated with hypoglycemia. Conversely, dialysates with higher dextrose concentrations are occasionally used in peritoneal dialysis to increase ultrafiltration, but this can lead to hyperglycemia.10,13
Thus, ESRD and hemodialysis exert opposing forces on insulin secretion, action, and metabolism, often creating unpredictable serum glucose values. For example, one would think that a patient who has insulin resistance would need more supplemental insulin; however, the reduced renal gluconeogenesis and insulin clearance seen in ESRD may result in variable net effects in different patients. In addition, ESRD and hemodialysis alter the pharmacokinetics of diabetic medications. Together, all of these factors contribute to wide fluctuations in glucose levels and increase the risk of hypoglycemic events.
HEMOGLOBIN A1c MAY BE FALSELY HIGH
Self-monitoring of blood glucose plus serial hemoglobin A1c measurements are the standard of care in diabetic patients without renal failure.
However, in diabetic patients with ESRD, elevated blood urea nitrogen causes formation of carbamylated hemoglobin, which is indistinguishable from glycosylated hemoglobin by electrical-charge-based assays and can cause the hemoglobin A1c measurement to be falsely elevated. Other factors such as the shorter red life span of red blood cells, iron deficiency, recent transfusion, and use of erythropoietin-stimulating agents may also cause underestimation of glucose control.14
Despite these limitations, the hemoglobin A1c level is considered a reasonable measure of glycemic control in ESRD. Glycated fructosamine and albumin are other measures of glycemic control with some advantages over hemoglobin A1c in dialysis patients. However, they are not readily available and can be affected by conditions that alter protein metabolism, including ESRD.15–18
Self-monitoring of blood glucose and continuous glucose monitoring systems provide real-time assessments of glycemic control, but both have limitations. Self-monitoring is subject to errors from poor technique, problems with the meters and strips, and lower sensitivity in measuring low blood glucose levels. Continuous monitoring is expensive and is less reliable at lower glucose concentrations, and thus it needs to be used in conjunction with other measures of glucose control. For these reasons, continuous glucose monitoring is not yet widely used.
The guidelines of the 2005 National Kidney Foundation Kidney Disease Outcomes Quality Initiative did not clearly establish a target hemoglobin A1c level for patients with diabetes and ESRD, but levels of 6% to 7% appear to be safe. The target fasting plasma glucose level should be lower than 140 mg/dL, and the target postprandial glucose level should be lower than 200 mg/dL.19
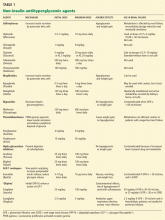
MOST ORAL DIABETES DRUGS ARE CONTRAINDICATED IN ESRD
Sulfonylureas
Sulfonylureas reduce blood glucose by stimulating the pancreatic beta cells to increase insulin secretion.
Sulfonylureas have a wide volume of distribution and are highly protein-bound,20 but only the unbound drug exerts a clinical effect. Because of protein binding, dialysis cannot effectively clear elevated levels of sulfonylurea drugs. Furthermore, many ESRD patients take drugs such as salicylates, sulfonamides, vitamin K antagonists, beta-blockers, and fibric acid derivatives, which may displace sulfonylureas from albumin, thus increasing the risk of severe hypoglycemia.
The first-generation sulfonylureas—chlorpropamide (Diabinese), acetohexamide (Dymelor), tolbutamide (Orinase), and tolazamide (Tolinase)—are almost exclusively excreted by the kidney and are therefore contraindicated in ESRD.21 Second-generation agents include glipizide (Glucotrol), glimepiride (Amaryl), glyburide (Micronase), and gliclazide (not available in the United States). Although these drugs are metabolized in the liver, their active metabolites are excreted in the urine, and so they should be avoided in ESRD.22
The only sulfonylurea recommended in ESRD is glipizide, which is also metabolized in the liver but has inactive or weakly active metabolites excreted in the urine. The suggested dose of glipizide is 2.5 to 10 mg/day. In ESRD, sustained-release forms should be avoided because of concerns of hypoglycemia.23
Meglitinides
The meglitinides repaglinide (Prandin) and nateglinide (Starlix) are insulin secretagogues that stimulate pancreatic beta cells. Like the sulfonylureas, nateglinide is hepatically metabolized, with renal excretion of active metabolites. Repaglinide, in contrast, is almost completely converted to inactive metabolites in the liver, and less than 10% is excreted by the kidneys.24,25 The meglitinides still pose a risk of hypoglycemia, especially in ESRD, and hence are not recommended for patients on hemodialysis.24,25
Biguanides
Metformin (Glucophage) is a biguanide that reduces hepatic gluconeogenesis and glucose output. It is excreted essentially unchanged in the urine and is therefore contraindicated in patients with renal disease due to the risks of bioaccumulation and lactic acidosis.22
Thiazolidinediones
The thiazolidinediones rosiglitazone (Avandia) and pioglitazone (Actos) are highly potent, selective agonists that work by binding to and activating a nuclear transcription factor, specifically, peroxisome proliferator-activated receptor gamma (PPAR-gamma). These drugs do not bioaccumulate in renal failure and so do not need dosing adjustments.26
The main adverse effect of these agents is edema, especially when they are combined with insulin therapy. Because of this effect, a joint statement of the American Diabetes Association and the American Heart Association recommends avoiding thiazolidinediones in patients in New York Heart Association (NYHA) class III or IV heart failure.27 Furthermore, caution is required in patients in compensated heart failure (NYHA class I or II) or in those at risk of heart failure, such as patients with previous myocardial infarction or angina, hypertension, left ventricular hypertrophy, significant aortic or mitral valve disease, age greater than 70 years, or diabetes for more than 10 years.27
In summary, although ESRD and dialysis do not affect the metabolism of thiazolidinediones, these agents are not recommended in ESRD because of the associated risk of fluid accumulation and precipitation of heart failure.
Alpha-glucosidase inhibitors
The alpha-glucosidase inhibitors acarbose (Precose) and miglitol (Glyset) slow carbohydrate absorption from the intestine. The levels of these drugs and their active metabolites are higher in renal failure,22 and since data are scarce on the use of these drugs in ESRD, they are contraindicated in ESRD.
GLP-1 ANALOGUES AND ‘GLIPTINS,’ NEW CLASSES OF DRUGS
Glucagon-like peptide-1 (GLP-1) stimulates glucose-dependent insulin release from pancreatic beta cells and inhibits inappropriate postprandial glucagon release. It also slows gastric emptying and reduces food intake. Dipeptidyl peptidase IV (DPP-IV) is an active ubiquitous enzyme that deactivates a variety of bioactive peptides, including GLP-1.
Exenatide (Byetta) is a naturally occurring GLP-1 analogue that is resistant to degradation by DPP-IV and has a longer half-life. Given subcutaneously, exenatide undergoes minimal systemic metabolism and is excreted in the urine.
No dose adjustment is required if the glomerular filtration rate (GFR) is greater than 30 mL/min, but exenatide is contraindicated in patients undergoing hemodialysis or in patients who have a GFR less than 30 mL/min (Table 1).
Sitagliptin (Januvia) is a DPP-IV inhibitor, or “gliptin,” that can be used as initial pharmacologic therapy for type 2 diabetes, as a second agent in those who do not respond to a single agent such as a sulfonylurea,28 metformin,29–31 or a thiazolidinedione,32 and as an additional agent when dual therapy with metformin and a sulfonylurea does not provide adequate glycemic control.28 Sitagliptin is not extensively metabolized and is mainly excreted in the urine.
The usual dose of sitagliptin is 100 mg orally once daily, with reduction to 50 mg for patients with a GFR of 30 to 50 mL/min, and 25 mg for patients with a GFR less than 30 mL/min.33 Sitagliptin may be used at doses of 25 mg daily in ESRD, irrespective of dialysis timing (Table 1).
Other drugs of this class are being developed. Saxagliptin (Onglyza) was recently approved by the US Food and Drug Administration and can be used at a dosage of 2.5 mg daily after dialysis.
Sitagliptin has been associated with gastrointestinal adverse effects. Anaphylaxis, angioedema, and Steven-Johnson syndrome have been reported. The risk of hypoglycemia increases when sitagliptin is used with sulfonylureas.
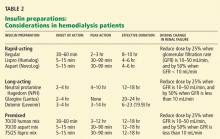
ESRD REDUCES INSULIN CLEARANCE
In healthy nondiabetic people, the pancreatic beta cells secrete half of the daily insulin requirement (about 0.5 units/kg/day) at a steady basal rate independent of glucose levels. The other half is secreted in response to prandial glucose stimulation.
Secreted into the portal system, insulin passes through the liver, where about 75% is metabolized, with the remaining 25% metabolized by the kidneys. About 60% of the insulin in the arterial bed is filtered by the glomerulus, and 40% is actively secreted into the nephric tubules.34 Most of the insulin in the tubules is metabolized into amino acids, and only 1% of insulin is secreted intact.
For diabetic patients receiving exogenous insulin, renal metabolism plays a more significant role since there is no first-pass metabolism in the liver. As renal function starts to decline, insulin clearance does not change appreciably, due to compensatory peritubular insulin uptake.35 But once the GFR drops below 20 mL/min, the kidneys clear markedly less insulin, an effect compounded by a decrease in the hepatic metabolism of insulin that occurs in uremia.36 Thus, despite the increase in insulin resistance caused by renal failure, the net effect is a reduced requirement for exogenous insulin in ESRD.37
Aisenpreis et al38 showed that the pharmacokinetic profile of insulin lispro (Humalog), which has a short onset of action and a short duration of action, may not only facilitate the correction of hyperglycemia but may also decrease the risk of late hypoglycemic episodes, which is of increased relevance in hemodialysis patients.
On the basis of the available evidence,39,40 we recommend a long-acting insulin such as insulin glargine (Lantus) or NPH insulin for basal requirements, along with a rapid-acting insulin analogue such as lispro or insulin aspart (NovoLog) before meals two or three times daily.
When the GFR drops to between 10 and 50 mL/min, the total insulin dose should be reduced by 25%; once the filtration rate is below 10 mL/min, as in ESRD, the insulin dose should be decreased by 50% from the previous amount.41,42
The newer insulins such as glargine and lispro are more favorable than NPH and regular insulin, but they cost more, which can be an obstacle for some patients.
OBSERVATIONS AND RECOMMENDATIONS
After reviewing the available evidence for the use of diabetic therapy in ESRD, we offer the following observations and recommendations:
- Glycemic control and monitoring in ESRD are complex.
- Patients with ESRD are especially susceptible to hypoglycemia, so diabetic drug therapy requires special caution.
- ESRD patients need ongoing diabetes education, with an emphasis on how to recognize and treat hypoglycemia.
- Diabetic pharmacotherapy in ESRD should be individualized. The targets of therapy are a hemoglobin A1c value between 6% and 7%, a fasting blood glucose level less than 140 mg/dL, and a postprandial glucose level less than 200 mg/dL.
- Of the oral antidiabetic drugs available, glipizide, sitagliptin, and saxagliptin may be used in ESRD. Glipizide, starting with 2.5 mg daily, should be reserved for ESRD patients with a hemoglobin A1c value less than 8.5%.
- Thiazolidinediones may cause fluid overload and thus should be avoided in ESRD.
- We recommend a long-acting insulin (glargine or NPH) for basal requirements, along with rapid-acting insulin before meals two or three times daily.
- The newer basal insulin (glargine) and rapid-acting insulin analogues (lispro or aspart insulin) are more favorable than NPH and regular insulin, but their higher cost could be an issue.
- Some patients may prefer a premixed insulin combination for convenience of dosing. In that case, NPH plus lispro insulin may be better than NPH plus regular insulin.
- For ESRD patients with type 1 diabetes, insulin therapy should be started at 0.5 IU/kg, which is half the calculated dose in patients without renal failure.
- For ESRD patients with type 2 diabetes, insulin therapy should be started at a total daily dose of 0.25 IU/kg.
- Further adjustments to the regimen should be individualized based on self-monitored blood glucose patterns.
- We recommend consulting an endocrinologist with expertise in managing diabetes in ESRD.
- National Institute of Diabetes and Digestive and Kidney Diseases: United States Renal Data System: USRDS 2005 Annual Data Report. Bethesda, MD: National Institutes of Health, 2005.
- Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997; 12:2105–2110.
- Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001; 24:909–913.
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002; 40:566–575.
- Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care 2006; 29:1496–1500.
- Williams ME, Lacson E, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006; 70:1503–1509.
- Tzamaloukas AH, Yuan ZY, Murata GH, Avasthi PS, Oreopoulos DG. Clinical associations of glycemic control in diabetics on CAPD. Adv Perit Dial 1993; 9:291–294.
- Tzamaloukas AH, Murata GH, Zager PG, Eisenberg B, Avasthi PS. The relationship between glycemic control and morbidity and mortality for diabetics on dialysis. ASAIO J 1993; 39:880–885.
- Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007; 30:1049–1055.
- Kovesdy C, Sharma K, Kalantar-Zadeh. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008; 52:766–777.
- Mak RH. Intravenous 1,25-dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int 1992; 41:1049–1054.
- Hajjar SM, Fadda GZ, Thanakitcharu P, Smogorzewski M, Massry SG. Reduced activity of Na(+)-K+ ATPase of pancreatic islet cells in chronic renal failure: role of secondary hyperparathyroidism. J Am Soc Nephrol 1992; 2:1355–1359.
- Grodstein GP, Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int 1981; 19:564–567.
- Joy MS, Cefali WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Lamb E, Venton TR, Cattell WR, Dawnay A. Serum glycated albumin and fructosamine in renal dialysis patients. Nephron 1993; 64:82–88.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem 1992; 29:437–442.
- Ford HC, Lim WC, Crooke MJ. Hemoglobin A1 and serum fructosamine levels in hyperthyroidism. Clin Chim Acta 1987; 166:317–321.
- Mak RH. Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial 2000; 13:4–8.
- Skillman TG, Feldman JM. The pharmacology of sulfonylureas. Am J Med 1981; 70:361–372.
- Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 2000; 35:500–505.
- Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial 2004; 17:365–370.
- United Kingdom Prospective Diabetes Study (UKPDS) 13. Relative efficacy of randomly allocated diet, sulphonylureas, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995; 310:83–88.
- Inoue T, Shibahara N, Miyagawa K, et al. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol 2003; 60:90–95.
- Nagai T, Imamura M, Iizuka K, Mori M. Hypoglycemia due to nateglinide administration in diabetic patient with chronic renal failure. Diabetes Res Clin Pract 2003; 59:191–194.
- Thompson-Culkin K, Zussman B, Miller AK, Freed MI. Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res 2002; 30:391–399.
- Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004; 27:256–263.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, et al; Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Carone FA, Peterson DR. Hydrolysis and transport of small peptides by the proximal tubule. Am J Physiol 1980; 238:F151–F158.
- Rabkin R, Simon NM, Steiner S, Colwell JA. Effects of renal disease on renal uptake and excretion of insulin in man. N Engl J Med 1970; 282:182–187.
- Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron 1992; 61:377–382.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Aisenpreis U, Pfützner A, Giehl M, Keller F, Jehle PM. Pharmacokinetics and pharmacodynamics of insulin Lispro compared with regular insulin in hemodialysis patients with diabetes mellitus. Nephrol Dial Transplant 1999; 14( suppl 4):5–6.
- Tunbridge FK, Newens A, Home PD, et al. A comparison of human ultralente- and lente-based twice-daily injection regimens. Diabet Med 1989; 6:496–501.
- Freeman SL, O’Brien PC, Rizza RA. Use of human ultralente as the basal insulin component in treatment of patients with IDDM. Diabetes Res Clin Pract 1991; 12:187–192.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26( suppl 4):73–85.
- Aronoff GR, Berns JS, Brier ME, et al, eds. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults, 4th ed. Philadelphia, PA: American College of Physicians, 1999.
Although diabetes is the most common cause of end-stage renal disease (ESRD) worldwide, accounting for 44.2% of ESRD patients in the US Renal Data System in 2005,1 data are scarce on how diabetes should best be treated in patients in ESRD.
We do know that blood glucose levels need to be well controlled in these patients. Several observational studies and one nonrandomized interventional study2–10 showed that higher levels of hemoglobin A1c were associated with higher death rates in patients with diabetes and chronic kidney disease after adjusting for markers of inflammation and malnutrition.
However, ESRD significantly alters glycemic control, the results of hemoglobin A1c testing, and the excretion of antidiabetic medications. The various and opposing effects of ESRD and dialysis can make blood glucose levels fluctuate widely, placing patients at risk of hypoglycemia—and presenting a challenge for nephrologists and internists.
In this review, we summarize the available evidence and make practical recommendations for managing diabetes in patients on hemodialysis.
GLUCOSE LEVELS MAY FLUCTUATE WIDELY
In ESRD, both uremia and dialysis can complicate glycemic control by affecting the secretion, clearance, and peripheral tissue sensitivity of insulin.
Several factors, including uremic toxins, may increase insulin resistance in ESRD, leading to a blunted ability to suppress hepatic gluconeogenesis and regulate peripheral glucose utilization. In type 2 diabetes without kidney disease, insulin resistance leads to increased insulin secretion. This does not occur in ESRD because of concomitant metabolic acidosis, deficiency of 1,25 dihydroxyvitamin D, and secondary hyperparathyroidism.11,12 Hemodialysis further alters insulin secretion, clearance, and resistance as the result of periodic improvement in uremia, acidosis, and phosphate handling.
The dextrose concentration in the dialysate can also affect glucose control. In general, dialysates with lower dextrose concentrations are used and may be associated with hypoglycemia. Conversely, dialysates with higher dextrose concentrations are occasionally used in peritoneal dialysis to increase ultrafiltration, but this can lead to hyperglycemia.10,13
Thus, ESRD and hemodialysis exert opposing forces on insulin secretion, action, and metabolism, often creating unpredictable serum glucose values. For example, one would think that a patient who has insulin resistance would need more supplemental insulin; however, the reduced renal gluconeogenesis and insulin clearance seen in ESRD may result in variable net effects in different patients. In addition, ESRD and hemodialysis alter the pharmacokinetics of diabetic medications. Together, all of these factors contribute to wide fluctuations in glucose levels and increase the risk of hypoglycemic events.
HEMOGLOBIN A1c MAY BE FALSELY HIGH
Self-monitoring of blood glucose plus serial hemoglobin A1c measurements are the standard of care in diabetic patients without renal failure.
However, in diabetic patients with ESRD, elevated blood urea nitrogen causes formation of carbamylated hemoglobin, which is indistinguishable from glycosylated hemoglobin by electrical-charge-based assays and can cause the hemoglobin A1c measurement to be falsely elevated. Other factors such as the shorter red life span of red blood cells, iron deficiency, recent transfusion, and use of erythropoietin-stimulating agents may also cause underestimation of glucose control.14
Despite these limitations, the hemoglobin A1c level is considered a reasonable measure of glycemic control in ESRD. Glycated fructosamine and albumin are other measures of glycemic control with some advantages over hemoglobin A1c in dialysis patients. However, they are not readily available and can be affected by conditions that alter protein metabolism, including ESRD.15–18
Self-monitoring of blood glucose and continuous glucose monitoring systems provide real-time assessments of glycemic control, but both have limitations. Self-monitoring is subject to errors from poor technique, problems with the meters and strips, and lower sensitivity in measuring low blood glucose levels. Continuous monitoring is expensive and is less reliable at lower glucose concentrations, and thus it needs to be used in conjunction with other measures of glucose control. For these reasons, continuous glucose monitoring is not yet widely used.
The guidelines of the 2005 National Kidney Foundation Kidney Disease Outcomes Quality Initiative did not clearly establish a target hemoglobin A1c level for patients with diabetes and ESRD, but levels of 6% to 7% appear to be safe. The target fasting plasma glucose level should be lower than 140 mg/dL, and the target postprandial glucose level should be lower than 200 mg/dL.19
MOST ORAL DIABETES DRUGS ARE CONTRAINDICATED IN ESRD
Sulfonylureas
Sulfonylureas reduce blood glucose by stimulating the pancreatic beta cells to increase insulin secretion.
Sulfonylureas have a wide volume of distribution and are highly protein-bound,20 but only the unbound drug exerts a clinical effect. Because of protein binding, dialysis cannot effectively clear elevated levels of sulfonylurea drugs. Furthermore, many ESRD patients take drugs such as salicylates, sulfonamides, vitamin K antagonists, beta-blockers, and fibric acid derivatives, which may displace sulfonylureas from albumin, thus increasing the risk of severe hypoglycemia.
The first-generation sulfonylureas—chlorpropamide (Diabinese), acetohexamide (Dymelor), tolbutamide (Orinase), and tolazamide (Tolinase)—are almost exclusively excreted by the kidney and are therefore contraindicated in ESRD.21 Second-generation agents include glipizide (Glucotrol), glimepiride (Amaryl), glyburide (Micronase), and gliclazide (not available in the United States). Although these drugs are metabolized in the liver, their active metabolites are excreted in the urine, and so they should be avoided in ESRD.22
The only sulfonylurea recommended in ESRD is glipizide, which is also metabolized in the liver but has inactive or weakly active metabolites excreted in the urine. The suggested dose of glipizide is 2.5 to 10 mg/day. In ESRD, sustained-release forms should be avoided because of concerns of hypoglycemia.23
Meglitinides
The meglitinides repaglinide (Prandin) and nateglinide (Starlix) are insulin secretagogues that stimulate pancreatic beta cells. Like the sulfonylureas, nateglinide is hepatically metabolized, with renal excretion of active metabolites. Repaglinide, in contrast, is almost completely converted to inactive metabolites in the liver, and less than 10% is excreted by the kidneys.24,25 The meglitinides still pose a risk of hypoglycemia, especially in ESRD, and hence are not recommended for patients on hemodialysis.24,25
Biguanides
Metformin (Glucophage) is a biguanide that reduces hepatic gluconeogenesis and glucose output. It is excreted essentially unchanged in the urine and is therefore contraindicated in patients with renal disease due to the risks of bioaccumulation and lactic acidosis.22
Thiazolidinediones
The thiazolidinediones rosiglitazone (Avandia) and pioglitazone (Actos) are highly potent, selective agonists that work by binding to and activating a nuclear transcription factor, specifically, peroxisome proliferator-activated receptor gamma (PPAR-gamma). These drugs do not bioaccumulate in renal failure and so do not need dosing adjustments.26
The main adverse effect of these agents is edema, especially when they are combined with insulin therapy. Because of this effect, a joint statement of the American Diabetes Association and the American Heart Association recommends avoiding thiazolidinediones in patients in New York Heart Association (NYHA) class III or IV heart failure.27 Furthermore, caution is required in patients in compensated heart failure (NYHA class I or II) or in those at risk of heart failure, such as patients with previous myocardial infarction or angina, hypertension, left ventricular hypertrophy, significant aortic or mitral valve disease, age greater than 70 years, or diabetes for more than 10 years.27
In summary, although ESRD and dialysis do not affect the metabolism of thiazolidinediones, these agents are not recommended in ESRD because of the associated risk of fluid accumulation and precipitation of heart failure.
Alpha-glucosidase inhibitors
The alpha-glucosidase inhibitors acarbose (Precose) and miglitol (Glyset) slow carbohydrate absorption from the intestine. The levels of these drugs and their active metabolites are higher in renal failure,22 and since data are scarce on the use of these drugs in ESRD, they are contraindicated in ESRD.
GLP-1 ANALOGUES AND ‘GLIPTINS,’ NEW CLASSES OF DRUGS
Glucagon-like peptide-1 (GLP-1) stimulates glucose-dependent insulin release from pancreatic beta cells and inhibits inappropriate postprandial glucagon release. It also slows gastric emptying and reduces food intake. Dipeptidyl peptidase IV (DPP-IV) is an active ubiquitous enzyme that deactivates a variety of bioactive peptides, including GLP-1.
Exenatide (Byetta) is a naturally occurring GLP-1 analogue that is resistant to degradation by DPP-IV and has a longer half-life. Given subcutaneously, exenatide undergoes minimal systemic metabolism and is excreted in the urine.
No dose adjustment is required if the glomerular filtration rate (GFR) is greater than 30 mL/min, but exenatide is contraindicated in patients undergoing hemodialysis or in patients who have a GFR less than 30 mL/min (Table 1).
Sitagliptin (Januvia) is a DPP-IV inhibitor, or “gliptin,” that can be used as initial pharmacologic therapy for type 2 diabetes, as a second agent in those who do not respond to a single agent such as a sulfonylurea,28 metformin,29–31 or a thiazolidinedione,32 and as an additional agent when dual therapy with metformin and a sulfonylurea does not provide adequate glycemic control.28 Sitagliptin is not extensively metabolized and is mainly excreted in the urine.
The usual dose of sitagliptin is 100 mg orally once daily, with reduction to 50 mg for patients with a GFR of 30 to 50 mL/min, and 25 mg for patients with a GFR less than 30 mL/min.33 Sitagliptin may be used at doses of 25 mg daily in ESRD, irrespective of dialysis timing (Table 1).
Other drugs of this class are being developed. Saxagliptin (Onglyza) was recently approved by the US Food and Drug Administration and can be used at a dosage of 2.5 mg daily after dialysis.
Sitagliptin has been associated with gastrointestinal adverse effects. Anaphylaxis, angioedema, and Steven-Johnson syndrome have been reported. The risk of hypoglycemia increases when sitagliptin is used with sulfonylureas.
ESRD REDUCES INSULIN CLEARANCE
In healthy nondiabetic people, the pancreatic beta cells secrete half of the daily insulin requirement (about 0.5 units/kg/day) at a steady basal rate independent of glucose levels. The other half is secreted in response to prandial glucose stimulation.
Secreted into the portal system, insulin passes through the liver, where about 75% is metabolized, with the remaining 25% metabolized by the kidneys. About 60% of the insulin in the arterial bed is filtered by the glomerulus, and 40% is actively secreted into the nephric tubules.34 Most of the insulin in the tubules is metabolized into amino acids, and only 1% of insulin is secreted intact.
For diabetic patients receiving exogenous insulin, renal metabolism plays a more significant role since there is no first-pass metabolism in the liver. As renal function starts to decline, insulin clearance does not change appreciably, due to compensatory peritubular insulin uptake.35 But once the GFR drops below 20 mL/min, the kidneys clear markedly less insulin, an effect compounded by a decrease in the hepatic metabolism of insulin that occurs in uremia.36 Thus, despite the increase in insulin resistance caused by renal failure, the net effect is a reduced requirement for exogenous insulin in ESRD.37
Aisenpreis et al38 showed that the pharmacokinetic profile of insulin lispro (Humalog), which has a short onset of action and a short duration of action, may not only facilitate the correction of hyperglycemia but may also decrease the risk of late hypoglycemic episodes, which is of increased relevance in hemodialysis patients.
On the basis of the available evidence,39,40 we recommend a long-acting insulin such as insulin glargine (Lantus) or NPH insulin for basal requirements, along with a rapid-acting insulin analogue such as lispro or insulin aspart (NovoLog) before meals two or three times daily.
When the GFR drops to between 10 and 50 mL/min, the total insulin dose should be reduced by 25%; once the filtration rate is below 10 mL/min, as in ESRD, the insulin dose should be decreased by 50% from the previous amount.41,42
The newer insulins such as glargine and lispro are more favorable than NPH and regular insulin, but they cost more, which can be an obstacle for some patients.
OBSERVATIONS AND RECOMMENDATIONS
After reviewing the available evidence for the use of diabetic therapy in ESRD, we offer the following observations and recommendations:
- Glycemic control and monitoring in ESRD are complex.
- Patients with ESRD are especially susceptible to hypoglycemia, so diabetic drug therapy requires special caution.
- ESRD patients need ongoing diabetes education, with an emphasis on how to recognize and treat hypoglycemia.
- Diabetic pharmacotherapy in ESRD should be individualized. The targets of therapy are a hemoglobin A1c value between 6% and 7%, a fasting blood glucose level less than 140 mg/dL, and a postprandial glucose level less than 200 mg/dL.
- Of the oral antidiabetic drugs available, glipizide, sitagliptin, and saxagliptin may be used in ESRD. Glipizide, starting with 2.5 mg daily, should be reserved for ESRD patients with a hemoglobin A1c value less than 8.5%.
- Thiazolidinediones may cause fluid overload and thus should be avoided in ESRD.
- We recommend a long-acting insulin (glargine or NPH) for basal requirements, along with rapid-acting insulin before meals two or three times daily.
- The newer basal insulin (glargine) and rapid-acting insulin analogues (lispro or aspart insulin) are more favorable than NPH and regular insulin, but their higher cost could be an issue.
- Some patients may prefer a premixed insulin combination for convenience of dosing. In that case, NPH plus lispro insulin may be better than NPH plus regular insulin.
- For ESRD patients with type 1 diabetes, insulin therapy should be started at 0.5 IU/kg, which is half the calculated dose in patients without renal failure.
- For ESRD patients with type 2 diabetes, insulin therapy should be started at a total daily dose of 0.25 IU/kg.
- Further adjustments to the regimen should be individualized based on self-monitored blood glucose patterns.
- We recommend consulting an endocrinologist with expertise in managing diabetes in ESRD.
Although diabetes is the most common cause of end-stage renal disease (ESRD) worldwide, accounting for 44.2% of ESRD patients in the US Renal Data System in 2005,1 data are scarce on how diabetes should best be treated in patients in ESRD.
We do know that blood glucose levels need to be well controlled in these patients. Several observational studies and one nonrandomized interventional study2–10 showed that higher levels of hemoglobin A1c were associated with higher death rates in patients with diabetes and chronic kidney disease after adjusting for markers of inflammation and malnutrition.
However, ESRD significantly alters glycemic control, the results of hemoglobin A1c testing, and the excretion of antidiabetic medications. The various and opposing effects of ESRD and dialysis can make blood glucose levels fluctuate widely, placing patients at risk of hypoglycemia—and presenting a challenge for nephrologists and internists.
In this review, we summarize the available evidence and make practical recommendations for managing diabetes in patients on hemodialysis.
GLUCOSE LEVELS MAY FLUCTUATE WIDELY
In ESRD, both uremia and dialysis can complicate glycemic control by affecting the secretion, clearance, and peripheral tissue sensitivity of insulin.
Several factors, including uremic toxins, may increase insulin resistance in ESRD, leading to a blunted ability to suppress hepatic gluconeogenesis and regulate peripheral glucose utilization. In type 2 diabetes without kidney disease, insulin resistance leads to increased insulin secretion. This does not occur in ESRD because of concomitant metabolic acidosis, deficiency of 1,25 dihydroxyvitamin D, and secondary hyperparathyroidism.11,12 Hemodialysis further alters insulin secretion, clearance, and resistance as the result of periodic improvement in uremia, acidosis, and phosphate handling.
The dextrose concentration in the dialysate can also affect glucose control. In general, dialysates with lower dextrose concentrations are used and may be associated with hypoglycemia. Conversely, dialysates with higher dextrose concentrations are occasionally used in peritoneal dialysis to increase ultrafiltration, but this can lead to hyperglycemia.10,13
Thus, ESRD and hemodialysis exert opposing forces on insulin secretion, action, and metabolism, often creating unpredictable serum glucose values. For example, one would think that a patient who has insulin resistance would need more supplemental insulin; however, the reduced renal gluconeogenesis and insulin clearance seen in ESRD may result in variable net effects in different patients. In addition, ESRD and hemodialysis alter the pharmacokinetics of diabetic medications. Together, all of these factors contribute to wide fluctuations in glucose levels and increase the risk of hypoglycemic events.
HEMOGLOBIN A1c MAY BE FALSELY HIGH
Self-monitoring of blood glucose plus serial hemoglobin A1c measurements are the standard of care in diabetic patients without renal failure.
However, in diabetic patients with ESRD, elevated blood urea nitrogen causes formation of carbamylated hemoglobin, which is indistinguishable from glycosylated hemoglobin by electrical-charge-based assays and can cause the hemoglobin A1c measurement to be falsely elevated. Other factors such as the shorter red life span of red blood cells, iron deficiency, recent transfusion, and use of erythropoietin-stimulating agents may also cause underestimation of glucose control.14
Despite these limitations, the hemoglobin A1c level is considered a reasonable measure of glycemic control in ESRD. Glycated fructosamine and albumin are other measures of glycemic control with some advantages over hemoglobin A1c in dialysis patients. However, they are not readily available and can be affected by conditions that alter protein metabolism, including ESRD.15–18
Self-monitoring of blood glucose and continuous glucose monitoring systems provide real-time assessments of glycemic control, but both have limitations. Self-monitoring is subject to errors from poor technique, problems with the meters and strips, and lower sensitivity in measuring low blood glucose levels. Continuous monitoring is expensive and is less reliable at lower glucose concentrations, and thus it needs to be used in conjunction with other measures of glucose control. For these reasons, continuous glucose monitoring is not yet widely used.
The guidelines of the 2005 National Kidney Foundation Kidney Disease Outcomes Quality Initiative did not clearly establish a target hemoglobin A1c level for patients with diabetes and ESRD, but levels of 6% to 7% appear to be safe. The target fasting plasma glucose level should be lower than 140 mg/dL, and the target postprandial glucose level should be lower than 200 mg/dL.19
MOST ORAL DIABETES DRUGS ARE CONTRAINDICATED IN ESRD
Sulfonylureas
Sulfonylureas reduce blood glucose by stimulating the pancreatic beta cells to increase insulin secretion.
Sulfonylureas have a wide volume of distribution and are highly protein-bound,20 but only the unbound drug exerts a clinical effect. Because of protein binding, dialysis cannot effectively clear elevated levels of sulfonylurea drugs. Furthermore, many ESRD patients take drugs such as salicylates, sulfonamides, vitamin K antagonists, beta-blockers, and fibric acid derivatives, which may displace sulfonylureas from albumin, thus increasing the risk of severe hypoglycemia.
The first-generation sulfonylureas—chlorpropamide (Diabinese), acetohexamide (Dymelor), tolbutamide (Orinase), and tolazamide (Tolinase)—are almost exclusively excreted by the kidney and are therefore contraindicated in ESRD.21 Second-generation agents include glipizide (Glucotrol), glimepiride (Amaryl), glyburide (Micronase), and gliclazide (not available in the United States). Although these drugs are metabolized in the liver, their active metabolites are excreted in the urine, and so they should be avoided in ESRD.22
The only sulfonylurea recommended in ESRD is glipizide, which is also metabolized in the liver but has inactive or weakly active metabolites excreted in the urine. The suggested dose of glipizide is 2.5 to 10 mg/day. In ESRD, sustained-release forms should be avoided because of concerns of hypoglycemia.23
Meglitinides
The meglitinides repaglinide (Prandin) and nateglinide (Starlix) are insulin secretagogues that stimulate pancreatic beta cells. Like the sulfonylureas, nateglinide is hepatically metabolized, with renal excretion of active metabolites. Repaglinide, in contrast, is almost completely converted to inactive metabolites in the liver, and less than 10% is excreted by the kidneys.24,25 The meglitinides still pose a risk of hypoglycemia, especially in ESRD, and hence are not recommended for patients on hemodialysis.24,25
Biguanides
Metformin (Glucophage) is a biguanide that reduces hepatic gluconeogenesis and glucose output. It is excreted essentially unchanged in the urine and is therefore contraindicated in patients with renal disease due to the risks of bioaccumulation and lactic acidosis.22
Thiazolidinediones
The thiazolidinediones rosiglitazone (Avandia) and pioglitazone (Actos) are highly potent, selective agonists that work by binding to and activating a nuclear transcription factor, specifically, peroxisome proliferator-activated receptor gamma (PPAR-gamma). These drugs do not bioaccumulate in renal failure and so do not need dosing adjustments.26
The main adverse effect of these agents is edema, especially when they are combined with insulin therapy. Because of this effect, a joint statement of the American Diabetes Association and the American Heart Association recommends avoiding thiazolidinediones in patients in New York Heart Association (NYHA) class III or IV heart failure.27 Furthermore, caution is required in patients in compensated heart failure (NYHA class I or II) or in those at risk of heart failure, such as patients with previous myocardial infarction or angina, hypertension, left ventricular hypertrophy, significant aortic or mitral valve disease, age greater than 70 years, or diabetes for more than 10 years.27
In summary, although ESRD and dialysis do not affect the metabolism of thiazolidinediones, these agents are not recommended in ESRD because of the associated risk of fluid accumulation and precipitation of heart failure.
Alpha-glucosidase inhibitors
The alpha-glucosidase inhibitors acarbose (Precose) and miglitol (Glyset) slow carbohydrate absorption from the intestine. The levels of these drugs and their active metabolites are higher in renal failure,22 and since data are scarce on the use of these drugs in ESRD, they are contraindicated in ESRD.
GLP-1 ANALOGUES AND ‘GLIPTINS,’ NEW CLASSES OF DRUGS
Glucagon-like peptide-1 (GLP-1) stimulates glucose-dependent insulin release from pancreatic beta cells and inhibits inappropriate postprandial glucagon release. It also slows gastric emptying and reduces food intake. Dipeptidyl peptidase IV (DPP-IV) is an active ubiquitous enzyme that deactivates a variety of bioactive peptides, including GLP-1.
Exenatide (Byetta) is a naturally occurring GLP-1 analogue that is resistant to degradation by DPP-IV and has a longer half-life. Given subcutaneously, exenatide undergoes minimal systemic metabolism and is excreted in the urine.
No dose adjustment is required if the glomerular filtration rate (GFR) is greater than 30 mL/min, but exenatide is contraindicated in patients undergoing hemodialysis or in patients who have a GFR less than 30 mL/min (Table 1).
Sitagliptin (Januvia) is a DPP-IV inhibitor, or “gliptin,” that can be used as initial pharmacologic therapy for type 2 diabetes, as a second agent in those who do not respond to a single agent such as a sulfonylurea,28 metformin,29–31 or a thiazolidinedione,32 and as an additional agent when dual therapy with metformin and a sulfonylurea does not provide adequate glycemic control.28 Sitagliptin is not extensively metabolized and is mainly excreted in the urine.
The usual dose of sitagliptin is 100 mg orally once daily, with reduction to 50 mg for patients with a GFR of 30 to 50 mL/min, and 25 mg for patients with a GFR less than 30 mL/min.33 Sitagliptin may be used at doses of 25 mg daily in ESRD, irrespective of dialysis timing (Table 1).
Other drugs of this class are being developed. Saxagliptin (Onglyza) was recently approved by the US Food and Drug Administration and can be used at a dosage of 2.5 mg daily after dialysis.
Sitagliptin has been associated with gastrointestinal adverse effects. Anaphylaxis, angioedema, and Steven-Johnson syndrome have been reported. The risk of hypoglycemia increases when sitagliptin is used with sulfonylureas.
ESRD REDUCES INSULIN CLEARANCE
In healthy nondiabetic people, the pancreatic beta cells secrete half of the daily insulin requirement (about 0.5 units/kg/day) at a steady basal rate independent of glucose levels. The other half is secreted in response to prandial glucose stimulation.
Secreted into the portal system, insulin passes through the liver, where about 75% is metabolized, with the remaining 25% metabolized by the kidneys. About 60% of the insulin in the arterial bed is filtered by the glomerulus, and 40% is actively secreted into the nephric tubules.34 Most of the insulin in the tubules is metabolized into amino acids, and only 1% of insulin is secreted intact.
For diabetic patients receiving exogenous insulin, renal metabolism plays a more significant role since there is no first-pass metabolism in the liver. As renal function starts to decline, insulin clearance does not change appreciably, due to compensatory peritubular insulin uptake.35 But once the GFR drops below 20 mL/min, the kidneys clear markedly less insulin, an effect compounded by a decrease in the hepatic metabolism of insulin that occurs in uremia.36 Thus, despite the increase in insulin resistance caused by renal failure, the net effect is a reduced requirement for exogenous insulin in ESRD.37
Aisenpreis et al38 showed that the pharmacokinetic profile of insulin lispro (Humalog), which has a short onset of action and a short duration of action, may not only facilitate the correction of hyperglycemia but may also decrease the risk of late hypoglycemic episodes, which is of increased relevance in hemodialysis patients.
On the basis of the available evidence,39,40 we recommend a long-acting insulin such as insulin glargine (Lantus) or NPH insulin for basal requirements, along with a rapid-acting insulin analogue such as lispro or insulin aspart (NovoLog) before meals two or three times daily.
When the GFR drops to between 10 and 50 mL/min, the total insulin dose should be reduced by 25%; once the filtration rate is below 10 mL/min, as in ESRD, the insulin dose should be decreased by 50% from the previous amount.41,42
The newer insulins such as glargine and lispro are more favorable than NPH and regular insulin, but they cost more, which can be an obstacle for some patients.
OBSERVATIONS AND RECOMMENDATIONS
After reviewing the available evidence for the use of diabetic therapy in ESRD, we offer the following observations and recommendations:
- Glycemic control and monitoring in ESRD are complex.
- Patients with ESRD are especially susceptible to hypoglycemia, so diabetic drug therapy requires special caution.
- ESRD patients need ongoing diabetes education, with an emphasis on how to recognize and treat hypoglycemia.
- Diabetic pharmacotherapy in ESRD should be individualized. The targets of therapy are a hemoglobin A1c value between 6% and 7%, a fasting blood glucose level less than 140 mg/dL, and a postprandial glucose level less than 200 mg/dL.
- Of the oral antidiabetic drugs available, glipizide, sitagliptin, and saxagliptin may be used in ESRD. Glipizide, starting with 2.5 mg daily, should be reserved for ESRD patients with a hemoglobin A1c value less than 8.5%.
- Thiazolidinediones may cause fluid overload and thus should be avoided in ESRD.
- We recommend a long-acting insulin (glargine or NPH) for basal requirements, along with rapid-acting insulin before meals two or three times daily.
- The newer basal insulin (glargine) and rapid-acting insulin analogues (lispro or aspart insulin) are more favorable than NPH and regular insulin, but their higher cost could be an issue.
- Some patients may prefer a premixed insulin combination for convenience of dosing. In that case, NPH plus lispro insulin may be better than NPH plus regular insulin.
- For ESRD patients with type 1 diabetes, insulin therapy should be started at 0.5 IU/kg, which is half the calculated dose in patients without renal failure.
- For ESRD patients with type 2 diabetes, insulin therapy should be started at a total daily dose of 0.25 IU/kg.
- Further adjustments to the regimen should be individualized based on self-monitored blood glucose patterns.
- We recommend consulting an endocrinologist with expertise in managing diabetes in ESRD.
- National Institute of Diabetes and Digestive and Kidney Diseases: United States Renal Data System: USRDS 2005 Annual Data Report. Bethesda, MD: National Institutes of Health, 2005.
- Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997; 12:2105–2110.
- Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001; 24:909–913.
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002; 40:566–575.
- Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care 2006; 29:1496–1500.
- Williams ME, Lacson E, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006; 70:1503–1509.
- Tzamaloukas AH, Yuan ZY, Murata GH, Avasthi PS, Oreopoulos DG. Clinical associations of glycemic control in diabetics on CAPD. Adv Perit Dial 1993; 9:291–294.
- Tzamaloukas AH, Murata GH, Zager PG, Eisenberg B, Avasthi PS. The relationship between glycemic control and morbidity and mortality for diabetics on dialysis. ASAIO J 1993; 39:880–885.
- Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007; 30:1049–1055.
- Kovesdy C, Sharma K, Kalantar-Zadeh. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008; 52:766–777.
- Mak RH. Intravenous 1,25-dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int 1992; 41:1049–1054.
- Hajjar SM, Fadda GZ, Thanakitcharu P, Smogorzewski M, Massry SG. Reduced activity of Na(+)-K+ ATPase of pancreatic islet cells in chronic renal failure: role of secondary hyperparathyroidism. J Am Soc Nephrol 1992; 2:1355–1359.
- Grodstein GP, Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int 1981; 19:564–567.
- Joy MS, Cefali WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Lamb E, Venton TR, Cattell WR, Dawnay A. Serum glycated albumin and fructosamine in renal dialysis patients. Nephron 1993; 64:82–88.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem 1992; 29:437–442.
- Ford HC, Lim WC, Crooke MJ. Hemoglobin A1 and serum fructosamine levels in hyperthyroidism. Clin Chim Acta 1987; 166:317–321.
- Mak RH. Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial 2000; 13:4–8.
- Skillman TG, Feldman JM. The pharmacology of sulfonylureas. Am J Med 1981; 70:361–372.
- Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 2000; 35:500–505.
- Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial 2004; 17:365–370.
- United Kingdom Prospective Diabetes Study (UKPDS) 13. Relative efficacy of randomly allocated diet, sulphonylureas, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995; 310:83–88.
- Inoue T, Shibahara N, Miyagawa K, et al. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol 2003; 60:90–95.
- Nagai T, Imamura M, Iizuka K, Mori M. Hypoglycemia due to nateglinide administration in diabetic patient with chronic renal failure. Diabetes Res Clin Pract 2003; 59:191–194.
- Thompson-Culkin K, Zussman B, Miller AK, Freed MI. Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res 2002; 30:391–399.
- Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004; 27:256–263.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, et al; Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Carone FA, Peterson DR. Hydrolysis and transport of small peptides by the proximal tubule. Am J Physiol 1980; 238:F151–F158.
- Rabkin R, Simon NM, Steiner S, Colwell JA. Effects of renal disease on renal uptake and excretion of insulin in man. N Engl J Med 1970; 282:182–187.
- Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron 1992; 61:377–382.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Aisenpreis U, Pfützner A, Giehl M, Keller F, Jehle PM. Pharmacokinetics and pharmacodynamics of insulin Lispro compared with regular insulin in hemodialysis patients with diabetes mellitus. Nephrol Dial Transplant 1999; 14( suppl 4):5–6.
- Tunbridge FK, Newens A, Home PD, et al. A comparison of human ultralente- and lente-based twice-daily injection regimens. Diabet Med 1989; 6:496–501.
- Freeman SL, O’Brien PC, Rizza RA. Use of human ultralente as the basal insulin component in treatment of patients with IDDM. Diabetes Res Clin Pract 1991; 12:187–192.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26( suppl 4):73–85.
- Aronoff GR, Berns JS, Brier ME, et al, eds. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults, 4th ed. Philadelphia, PA: American College of Physicians, 1999.
- National Institute of Diabetes and Digestive and Kidney Diseases: United States Renal Data System: USRDS 2005 Annual Data Report. Bethesda, MD: National Institutes of Health, 2005.
- Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997; 12:2105–2110.
- Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001; 24:909–913.
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002; 40:566–575.
- Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care 2006; 29:1496–1500.
- Williams ME, Lacson E, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006; 70:1503–1509.
- Tzamaloukas AH, Yuan ZY, Murata GH, Avasthi PS, Oreopoulos DG. Clinical associations of glycemic control in diabetics on CAPD. Adv Perit Dial 1993; 9:291–294.
- Tzamaloukas AH, Murata GH, Zager PG, Eisenberg B, Avasthi PS. The relationship between glycemic control and morbidity and mortality for diabetics on dialysis. ASAIO J 1993; 39:880–885.
- Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007; 30:1049–1055.
- Kovesdy C, Sharma K, Kalantar-Zadeh. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008; 52:766–777.
- Mak RH. Intravenous 1,25-dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int 1992; 41:1049–1054.
- Hajjar SM, Fadda GZ, Thanakitcharu P, Smogorzewski M, Massry SG. Reduced activity of Na(+)-K+ ATPase of pancreatic islet cells in chronic renal failure: role of secondary hyperparathyroidism. J Am Soc Nephrol 1992; 2:1355–1359.
- Grodstein GP, Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int 1981; 19:564–567.
- Joy MS, Cefali WT, Hogan SL, Nachman PH. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297–307.
- Lamb E, Venton TR, Cattell WR, Dawnay A. Serum glycated albumin and fructosamine in renal dialysis patients. Nephron 1993; 64:82–88.
- Inaba M, Okuno S, Kumeda Y, et al; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896–903.
- Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem 1992; 29:437–442.
- Ford HC, Lim WC, Crooke MJ. Hemoglobin A1 and serum fructosamine levels in hyperthyroidism. Clin Chim Acta 1987; 166:317–321.
- Mak RH. Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial 2000; 13:4–8.
- Skillman TG, Feldman JM. The pharmacology of sulfonylureas. Am J Med 1981; 70:361–372.
- Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 2000; 35:500–505.
- Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial 2004; 17:365–370.
- United Kingdom Prospective Diabetes Study (UKPDS) 13. Relative efficacy of randomly allocated diet, sulphonylureas, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995; 310:83–88.
- Inoue T, Shibahara N, Miyagawa K, et al. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol 2003; 60:90–95.
- Nagai T, Imamura M, Iizuka K, Mori M. Hypoglycemia due to nateglinide administration in diabetic patient with chronic renal failure. Diabetes Res Clin Pract 2003; 59:191–194.
- Thompson-Culkin K, Zussman B, Miller AK, Freed MI. Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res 2002; 30:391–399.
- Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004; 27:256–263.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, et al; Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30:1862–1864.
- Carone FA, Peterson DR. Hydrolysis and transport of small peptides by the proximal tubule. Am J Physiol 1980; 238:F151–F158.
- Rabkin R, Simon NM, Steiner S, Colwell JA. Effects of renal disease on renal uptake and excretion of insulin in man. N Engl J Med 1970; 282:182–187.
- Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron 1992; 61:377–382.
- Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003; 20:642–645.
- Aisenpreis U, Pfützner A, Giehl M, Keller F, Jehle PM. Pharmacokinetics and pharmacodynamics of insulin Lispro compared with regular insulin in hemodialysis patients with diabetes mellitus. Nephrol Dial Transplant 1999; 14( suppl 4):5–6.
- Tunbridge FK, Newens A, Home PD, et al. A comparison of human ultralente- and lente-based twice-daily injection regimens. Diabet Med 1989; 6:496–501.
- Freeman SL, O’Brien PC, Rizza RA. Use of human ultralente as the basal insulin component in treatment of patients with IDDM. Diabetes Res Clin Pract 1991; 12:187–192.
- Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000; 26( suppl 4):73–85.
- Aronoff GR, Berns JS, Brier ME, et al, eds. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults, 4th ed. Philadelphia, PA: American College of Physicians, 1999.
KEY POINTS
- Blood glucose levels can fluctuate widely due to various and opposing effects of ESRD and dialysis.
- The hemoglobin A1c level can be falsely high in ESRD, but it is still a reasonable measure of glycemic control in this population.
- Most diabetes drugs are excreted at least in part by the kidney, so that patients in ESRD are at greater risk of hypoglycemia.
- Insulin is the cornerstone of treatment, since most oral diabetes drugs are contraindicated or not recommended in this population. Insulin doses should be lowered in those with low glomerular filtration rates.
Surgical Comanagement
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
Brief Report
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.
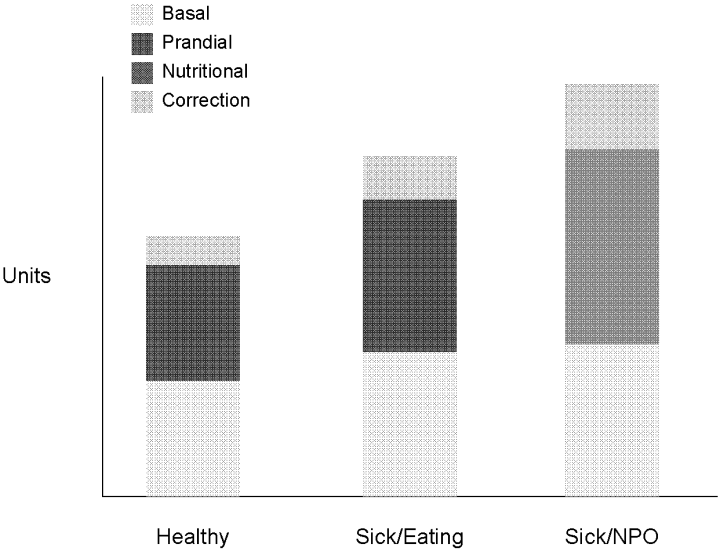
| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.

| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
Tight glycemic control in the hospitalized patient is not a simple task. Hospitalized patients are characterized by high levels of counterregulatory hormones (catecholamines, cortisol, and growth hormone) and cytokines that vary greatly in the context of sepsis, burns, hypoxia, cardiovascular disease, pain, surgery, and trauma. In addition, inpatients have unpredictable eating times and little to no physical activity. Each of the major classes of oral glycemic agents has significant limitations for inpatient use and provides little flexibility or opportunity for titration in a setting where acute changes demand these qualities. As a result, sliding‐scale insulin (SSI) regimens are often used to treat hyperglycemia in patients with or without diabetes in these clinical situations.
SSI usually consists of rapid‐acting or regular insulin ordered in a specified number of units for a given degree of hyperglycemia without regard to the timing of food, any preexisting insulin administration, or even individualization of a patient's sensitivity to insulin. This is not a physiologic approach to insulin management and not an ideal strategy for managing hyperglycemia. Because many SSI regimens do not initiate therapy until the blood glucose level is more than 200 mg/dL, SSI uses hyperglycemia as a threshold. This allows hyperglycemia to persist for long periods without intervention. In turn, SSI is reactive instead of proactive. With SSI, the current dose of insulin is based on the inadequacy of the previous dose, creating a chase‐your‐tail phenomenon. In addition, once the SSI regimen begins, glycemic control is rarely assessed by a physician until blood glucose is dangerously low or high (<60 or >400 mg/dL). Finally, SSI provides no basal insulin. Hospitalized patients with stress‐induced hyperglycemia require not only postprandial insulin but also basal insulin to control blood glucose between meals and at night.
Evidence supporting SSI as a primary method of blood glucose control in diabetic patients is lacking. A search of MEDLINE for the period from 1966 to 2003 with the terms sliding scale insulin, sliding scale, and sliding combined with insulin yielded a total of 52 publications, none of which showed a benefit of sliding‐scale insulin in improving glycemic control or clinical outcomes. Retrospective and nonrandomized studies confirmed that SSI is associated with more hyper‐ and hypoglycemia with longer hospital stays.13 Queale et al. published the largest prospective cohort study (n = 171) of diabetic patients on SSI.4 More than 40% had at least one episode of hyperglycemia (>300 mg/dL), and 25% had more than one episode. Use of SSI alone increased the likelihood of hyperglycemia 3‐fold. Hypoglycemia occurred in 23%. Despite this poor performance in controlling blood glucose, the SSI remained unadjusted throughout the hospital stay for more than 80% of patients. In total, the clinical studies and clinical reviews on SSI confirmed that it is an inappropriate approach to blood glucose control in diabetic patients. Yet, SSI use in the inpatient setting continues to be a routine passed down from attending physicians to residents and medical students. In one recent study, 61% of diabetic patients admitted to the hospital for reasons other than metabolic control were on SSI.5 This sliding‐scale culture tolerates hyperglycemia and relieves the burden on the medical team to closely manage the glucose. Clinicians rely on the SSI to manage hyperglycemia rather than make frequent insulin adjustments.
Insulin, given either intravenously as a continuous infusion or subcutaneously, is the most effective agent for achieving glycemic control in hospitalized patients. Intravenous insulin infusions have been used for many years and have a proven track record for efficacy and safety. It does require frequent bedside blood glucose monitoring, which may limit its use on regular medical floors. The ideal frequency for monitoring has not been studied, but it is generally recommended that blood glucose be tested every hour until a stable infusion rate is reached. Unlike SSI, effective subcutaneous insulin therapy should define the dose components physiologically in the form of basal, nutritional or prandial, and correction doses (Fig. 1). Basal insulin is a patient's baseline level of insulin available throughout the day. Basal insulin gives the patient enough insulin to suppress hepatic glucose output, and it keeps the body from becoming hyperglycemic and ketoacidotic when not eating. Nutritional insulin is defined as the insulin needed to cover any intravenous glucose the patient is receiving, intravenous or enteral alimentation, and calories consumed in meals. If the patient is eating and is not receiving any other sources of calories, nutritional insulin would be the same as prandial insulin. In addition to basal and nutritional insulin requirements, patients often require supplemental or correction doses of insulin to treat unexpected hyperglycemia. Therefore, subcutaneous insulin can be given as a scheduled or programmed dose (basal + nutritional) and then a rapid‐acting supplemental (correction) dose to cover any hyperglycemia above target. The supplemental dose should not be confused with SSI, which does not provide any programmed basal and nutritional insulin. To provide the right amounts of basal and prandial insulin, you need to choose from the available therapies by examining their properties (Table 1). The ideal basal insulin should be long acting without identifiable peaks in concentration. For patients who are not eating, nutritional doses can be programmed with intermediate‐acting insulin. When giving insulin to patients before meals, rapid‐acting insulin analogs are best suited for the hospitalized patient because of their short onset of action. Regular insulin is also short acting, but it takes 30 minutes to take effect; thus, the dose needs to be timed at least a half hour prior to the meal. In addition, regular insulin can last for 6‐8 hours if large doses are used, which is not an ideal quality to have if trying to control postprandial glucose. The best way to mimic normal physiology is to use a combination of several types of insulin. A common strategy is to give a single daily injection of basal insulin (glargine/detimir) and then use rapid‐acting insulin analogs (lispro/aspart/glulisine) to cover prandial and correction doses.

| Time to Action | Peak | Duration | |
|---|---|---|---|
| Lispro/aspart/glulisine | 5‐15 minutes | 1‐2 hours | 3‐6 hours |
| Human NPH | 1‐2 hours | 4‐8 hours | 10‐20 hours |
| Regular/human | 30‐60 minutes | 2‐4 hours | 6‐10 hours |
| Glargine/detimir | 1‐2 hours | Flat | 24 hours |
The initial doses of scheduled subcutaneous insulin are based on previously established dose requirements, previous experience of the same patient during similar circumstances, requirements during a stable continuous insulin infusion, and/or knowledge of how stable medical condition and nutritional intake are. For patients whose insulin requirements are unknown and whose nutritional intake will be adequate, a reasonable assumption based on body weight is 0.5‐0.7 units/kg per 24 hours. Type 2 diabetics may need more, however; regardless, the patient's regimen should be started low and worked up to the dose to meet the demonstrated need. For type 1 diabetics with limited nutritional intake, the amount of scheduled insulin calculated by body weight should be reduced by 50%. For type 2 diabetics with limited nutritional intake, endogenous insulin may be adequate for basal requirements, and until results of monitoring indicate a further need for scheduled insulin, only correction doses should be used initially.
Many patients will need to transition from intravenous to subcutaneous insulin therapy when transferred from the critical care unit to the regular nursing floor. To maintain effective blood levels of insulin, it is necessary to administer short‐ or rapid‐acting insulin subcutaneously 1‐2 hours before or intermediate‐ or long‐acting insulin 2‐3 hours before stopping the insulin infusion. Subcutaneous insulin with an appropriate duration of action may be administered as a single dose or repeatedly to maintain basal effect until the time of day when insulin or analog, whichever preferred for basal effect, normally would be provided. For example, patients who typically receive glargine at night but have their insulin infusion stopped at lunchtime could receive a one‐time dose of NPH before interruption of the insulin infusion.
Hypoglycemia is a concern in hospitalized patients with diabetes, and it has been a major barrier to aggressive treatment of hyperglycemia in the hospital. Yet hypoglycemia can be predicted and prevented. Factors that increase the risk of hypoglycemia in the hospital include inadequate glucose monitoring; lack of clear communication or coordination between dietary, transportation, and nursing staff; and illegible orders.6 Clear algorithms for insulin orders and clear hypoglycemia protocols will reduce the likelihood of severe hypoglycemia occurring.
Although most positive outcomes associated with the new glycemic targets are derived from the critical care setting, there is a rationale supporting their benefit for other patients. The current glycemic targets for hospitalized patients warrant an approach that stresses the use of insulin in a way that matches normal physiology. The traditional SSI regimen is ineffective, and using it to manage glucose in the inpatient setting can no longer be justified.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.
- ,,,,.Efficacy of sliding‐scale insulin therapy: a comparison with prospective regimens.Fam Pract Res J.1994;14:313–321.
- ,,,,.Causes of hyperglycemia and hypoglycemia in adult inpatients.Am J Health Syst Pharm.2005;62:714–719.
- ,.Sliding‐scale insulin: an antiquated approach to glycemic control in hospitalized patients.Am J Health Syst Pharm.2004;61:1611–1614.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- .Hospital management of diabetes: beyond the sliding scale.Cleve Clin J Med.2004;71:801–808.
Tight Glycemic Control / Michota and Braithwaite
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.