User login
Clinical Progress Note: Myocardial Injury After Noncardiac Surgery
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
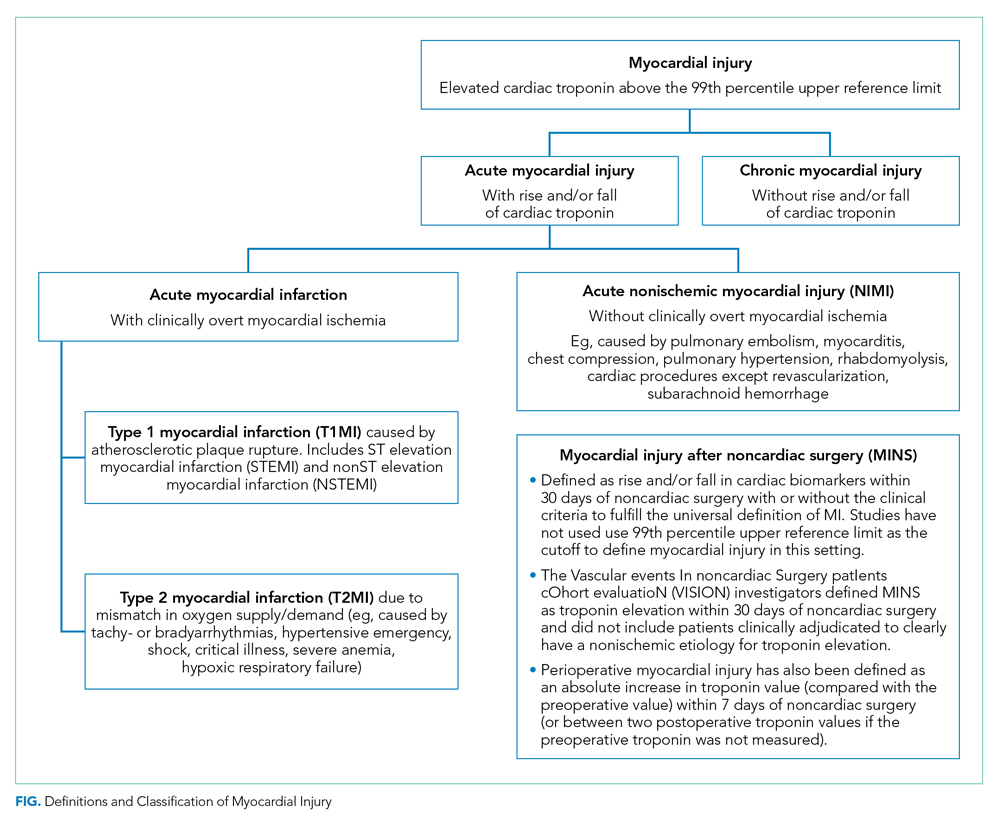
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8
EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
© 2020 Society of Hospital Medicine
Should we stop aspirin before noncardiac surgery?
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
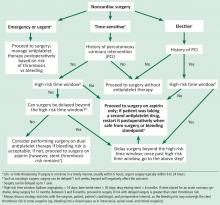
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine
Perioperative medication management: General principles and practical applications
As a hospitalist who practices in a perioperative clinic, I probably spend more of my time with patients reviewing and discussing the medications they are taking than on any other single subject. Surgical patients—many of whom are elderly—commonly are on multiple medications, have renal or hepatic disease that can alter drug metabolism, and may not be adequately educated about their medication regimens.
Patient safety is the overriding concern behind perioperative medication management, consistent with the medication-related objectives in the Joint Commission’s 2009 National Patient Safety Goals.1 The increasing surgical burden that comes with an aging population, along with rising expectations for functional recovery, has likewise elevated the importance of perioperative medication management.
Despite these demands, there is scant evidence from randomized controlled trials to directly guide perioperative medication management. For this reason, recommendations in this area rely largely on other forms of evidence, including expert consensus, case reports, in vitro studies, recommendations from pharmaceutical companies, and other known data (pharmacokinetics, drug interactions with anesthetic agents, and effects of the agent on the primary disease and on perioperative risk).
This article reviews general principles of perioperative medication management and then presents four case vignettes to explore perioperative recommendations for a number of common medication classes. It is not intended as a comprehensive review of the perioperative management of all medications, as numerous classes (antiplatelets, beta-blockers, oral hypogycemic agents, insulin, statins) are discussed in detail elsewhere in this proceedings supplement.
GENERAL CONSIDERATIONS IN MEDICATION MANAGEMENT
A comprehensive medication history is fundamental
Effective perioperative management of medications requires an understanding of the patient and his or her comorbidities so that the risk of perioperative decompensation can be gauged. This understanding stems from a thorough medical history that includes a comprehensive medication history to provide a complete inventory of the following:
- All prescription medications
- All over-the-counter (OTC) agents (including nonsteroidal anti-inflammatory drugs [NSAIDs])
- All vitamins
- All herbal medications.
When to stop, when to resume?
Guidance on stopping and resuming medications in the perioperative period is relatively absent from the literature. General considerations include the following:
- The potential for withdrawal when stopping a medication
- The progression of disease with interruption of drug therapy
- The potential for interactions with anesthetic agents if the medication is continued.
Withdrawal potential
Abrupt discontinuation of some drugs may lead to unnecessary complications due to the potential for withdrawal. Common medications that have been associated with withdrawal symptoms are selective serotonin reuptake inhibitors (SSRIs), beta-blockers, clonidine, statins, and corticosteroids.2 A recent systematic literature review concluded that continuation of chronic corticosteroid therapy without supplemental (stress) doses of corticosteroids is appropriate unless patients have primary disease of the hypothalamic-pituitary-adrenal axis, in which case perioperative stress dosing is recommended to avoid acute adrenal insufficiency (addisonian crisis).3
Patients on chronic drugs are more likely to have complications
In a medication survey of 1,025 patients admitted to a general surgery unit, Kennedy et al reported that 49% of the patients were taking medications (other than vitamins) unrelated to their surgical procedure.4 Even while this percentage is considerably lower than what I observe in my practice, this study showed that medication use has important perioperative consequences4:
- The odds ratio for a postoperative complication was 2.7 (95% CI, 1.76–4.04) if patients were taking a drug unrelated to their surgery.
- The risk of a complication was particularly elevated if patients were taking cardiovascular drugs or agents that act on the central nervous system; if patients were on NPO (“nothing by mouth”) orders for more than 24 hours before surgery; and if the operation was more than 1 hour in duration. These findings could reflect destabilization of the disease processes for which the patients were taking chronic medications that required interruption.
Unintended discontinuation of chronic drugs
Stopping a chronic medication for a surgical procedure raises the possibility that its resumption could be overlooked, especially since medical errors are particularly common in the transition between health care settings following hospital discharge. A population-based cohort study among all elderly patients discharged from Ontario, Canada, hospitals over a 5½-year period found that 11.4% of patients undergoing elective surgery did not resume their indicated chronic warfarin therapy within 6 months after its presurgical discontinuation.5 Although 6-month rates of unintended failure to resume therapy were lower for statins (4%) and ophthalmic beta-blocker drops (8%),5 these findings underscore that drug discontinuation always carries a risk that therapy might not be resumed as indicated.
Additional considerations
Stress response to surgery. Decisions about perioperative drug therapy should always take into account the stress response to surgery and the challenge it presents to homeostasis in the face of increased sympathetic tone and release of pituitary hormones.
Unreliable absorption of oral medications. Surgery and the postoperative state can lead to unreliable absorption of oral drugs for any of a number of reasons: villous atrophy, diminished blood flow to the gut, edema, mucosal ischemia, diminished motility from postoperative ileus, and use of narcotics.6
Take-away general principles
The following principles can be applied to guide perioperative medication management in a general sense7:
- Continue medications with withdrawal potential
- Discontinue medications that increase surgical risk and are not essential for short-term quality of life
- Use clinical judgment when neither of the above two principles applies, but be mindful that many other medications are given in the narrow perioperative time window and that metabolism and elimination of chronic drugs may be altered.
CASE 1: A PATIENT ON A NONPRESCRIPTION NSAID FOR SEVERE ARTHRITIS
A 55-year-old man with severe osteoarthritis is scheduled for total hip arthroplasy in 2 days. He stopped his aspirin (325 mg/day) 1 week ago but continued taking ibuprofen 600 three times daily with food, explaining that “no one told me to stop.” His last dose was yesterday evening.
Question: What should you do?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop the ibuprofen now
C. Check his bleeding time and proceed if normal
D. Just tell the patient to stop the ibuprofen now
E. Proceed to the operating room regardless of the ibuprofen dose
The best approach would be to notify the surgeon and tell the patient to stop the ibuprofen now. NSAIDs such as ibuprofen reversibly inhibit platelet cyclooxygenase (COX), diminish thromboxane A2 production, diminish platelet aggregation, and can increase bleeding time measurement and overall bleeding risk. They can induce renal failure in combination with other drugs, especially in the setting of hypotension.8,9 COX-2 inhibitors have less effect on platelet function but retain the potential for renal toxicity and also confer well-known cardiovascular risks.
In the past, NSAIDs were typically held for 7 days before surgery, but this practice was not supported with much evidence. In vitro assessment indicates that platelet function normalizes within 24 hours after cessation of regular ibuprofen or dexibuprofen in healthy individuals.10,11
Since NSAIDs vary in their effect on bleeding time, which does not correlate well with elimination half-life, a general recommendation is to stop most NSAIDs at least 3 days before surgery.
CASE 2: A PATIENT ON MULTIPLE CARDIOVASCULAR DRUGS
A 67-year-old man with dilated cardiomyopathy and an ejection fraction of 25% (well compensated) is scheduled for a laparoscopic cholecystectomy tomorrow. He is taking lisinopril (40 mg/day), irbesartan (150 mg/day), and furosemide (80 mg/day).
Question: What is your advice?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop his medications now
C. Hold all of the above medications on the morning of surgery
D. Proceed to the operating room with the usual doses of his medications on the morning of surgery
The best approach is to withhold these medications on the morning of surgery.
Diuretics are typically held on the morning of surgery because of the potential for hypovolemia and electrolyte depletion.
Angiotensin-converting enzyme (ACE) inhibitors intensify the hypotensive effects of anesthesia induction. Because angiotensin II plays a key role in maintaining circulating volume in response to stressors, volume deficits can occur in ACE inhibitor-treated patients as angiotensin II cannot compensate for venous pooling of blood, resulting in diminished cardiac output and arterial hypotension. However, continued renin-angiotensin system suppression may protect regional circulation, as has been demonstrated by reduced release of cardiac enzymes with ACE inhibitor continuation (compared with interruption) in cardiac surgery patients. ACE inhibitors also have a renal protective effect, preserving glomerular filtration rate in patients undergoing aortic abdominal aneurysm repair or coronary artery bypass graft surgery. Hypotension with ACE inhibition is treatable with sympathomimetics, alpha-agonists, and intravenous fluids.12–15
If a patient’s ACE inhibitor is stopped, be prepared for rebound postoperative hypertension. The probability of postoperative atrial fibrillation is also increased with ACE inhibitor interruption.14 In patients with left ventricular dysfunction undergoing noncardiac vascular surgery, continued ACE inhibition is associated with reduced mortality.16 These data argue, at the very least, for prompt resumption of ACE inhibitors after surgery.
Angiotensin receptor blockers (ARBs) have largely the same clinical benefits as do ACE inhibitors. These agents also increase the risk of hypotension upon induction of anesthesia, and this hypotension is not as responsive to conventional vasopressors such as ephedrine and phenylephrine; a better response is achieved with vasopressin.15 In light of the long half-life of ARBs, current thinking is to withhold them 24 hours before surgery.
Rosenman et al recently published a meta-analysis of five studies assessing the effects of continuing or withholding ACE inhibitors and ARBs in the preoperative period.17 They found a statistically significant increase in the incidence of perioperative hypotension in patients in whom the drugs were continued compared with those in whom the drugs were withheld (relative risk = 1.50; 95% CI, 1.15–1.96), but there was no significant difference in the rate of perioperative MI between the two groups. Notably, the indication for ACE inhibitor or ARB use in all of the studies was hypertension, not heart failure.
My approach to the perioperative management of ACE inhibitors and ARBs is to withhold them on the morning of surgery (in the case of ARBs, 24 hours prior to surgery) if their only indication is for hypertension and if the patient’s blood pressure is well controlled. If the patient has another indication for these agents or has hypertension that is not well controlled, I am inclined to continue these agents but will first discuss the decision with the anesthesiologist.
CASE 3: A PATIENT TAKING HERBAL MEDICATIONS
A 68-year-old woman with a history of hypertension, osteoarthritis, and osteoporosis is scheduled for total hip replacement in 7 days. Her medications include atenolol, hydrochlorothiazide, and alendronate. She also reports taking some natural herbal medications. She does not recall their names initially but calls back with the names: ginkgo biloba for her memory and echinacea for her immune system.
Question: What are your recommendations?
A. Stop all medications now except atenolol and proceed to surgery
B. Stop the herbals now but take all other medications on the morning of surgery
C. Stop the herbals now and take only atenolol on the morning of surgery
D. Continue all medications now and take atenolol and the herbals on the morning of surgery
E. Cancel the surgery and call an herbalist for guidance
The best strategy is to stop the herbals now and tell her to take only atenolol (a beta-blocker) on the morning of surgery.
Because the US Food and Drug Administration (FDA) does not regulate herbal products, the contents of these products can vary widely. For example, an analysis using mass spectrometry of 50 commercial ginseng products from 11 countries found that the ginseng content varied from 0% (six preparations) to 9%.19 Catecholamine-type compounds were found in some of the products.19
Because of the uncertainty over their actual contents, herbal medications should be stopped at least 7 days prior to surgery. If a patient is still taking herbal supplements on the day before surgery, I typically alert the anesthesiologist and surgeon.
CASE 4: A PATIENT ON MULTIPLE PSYCHOTROPICS
A 38-year-old woman with a history of severe major depression is scheduled for a mastectomy for breast cancer the next day. Her medications include fluoxetine, lorazepam, and phenelzine, all of which she has been taking for many years.
Question: What is your course of action?
A. Call the surgeon and cancel the surgery
B. Call the surgeon and notify the day-of-surgery anesthesiologist that the patient is taking these agents
C. Stop all the medications now and proceed to the operating room
D. Request a psychiatric consult for an alternative drug regimen
E. Proceed and advise the patient to take all of these agents on the morning of surgery
My approach would be to notify the day-of-surgery anesthesiologist, specifically about the phenelzine, which is a monoamine oxidase (MAO) inhibitor (see below). The other two agents can be taken on the morning of surgery, although fluoxetine has a long half-life, so missing a dose should not be problematic, and lorazepam can be given intravenously if needed.
SSRIs, including fluoxetine, are generally safe perioperatively. Serotonin depletion from platelets, however, increases the risk of bleeding, especially gastrointestinal bleeding, when SSRIs are used with NSAIDs.20–22 A neurosurgical procedure may therefore be especially risky in patients who have not stopped their SSRI if they are also taking an NSAID or an herbal medication that may increase the risk of bleeding. The caveat to stopping SSRIs is the potential for a minor withdrawal syndrome.
Tricyclic antidepressants inhibit the reuptake of norepinephrine and serotonin and may increase the action of sympathomimetics. Although arrhythmias are thought to be a concern with tricyclics, there are no reported cases of association in the literature. In general, I advise continuing triclyclics perioperatively, especially in patients who are on high doses.
Benzodiazepines, including lorazepam, are safe to use perioperatively, and a potential for withdrawal symptoms (hypertension, agitation, delirium, seizures) argues against their discontinuation. Chronic benzodiazepine use may increase anesthetic requirements.
Antipsychotic agents, which include haloperidol, olanzapine, risperidone, and ziprasidone, have multiple routes of administration—intramuscular, oral, sublingual, and intravenous. These agents are generally safe to use in the perioperative period.
MAO inhibitors, including phenelzine, are no longer commonly used and are typically reserved for the treatment of refractory depression. But they merit attention, as their use can cause accumulation of biogenic amines in the central and autonomic nervous systems. There are two types of MAO reactions—excitatory and depressive. Excitatory reactions lead to serotonin syndrome. Depressive reactions induce inhibition of hepatic microsomal enzymes, leading to narcotic accumulation and increased sedation.23
MAO inhibitors are also of concern because of their many drug interactions. When used with indirect sympathomimetics such as ephedrine, they promote a massive release of stored norepinephrine, leading to severe hypertension. When used with opioids like meperidine and dextromethorphan, MAO inhibitors are associated with a serotonin syndrome characterized by agitation, headache, fever, seizures, coma, and death.
Discontinuing MAO inhibitors before the day of surgery is no longer universally recommended, due to the risk of precipitating an exacerbation of major depression. Safe anesthetic regimens in the setting of MAO inhibitors involve avoidance of meperidine (morphine and fentanyl are safe) and use of only direct-acting sympathomimetics.
CONCLUSIONS
A good medication history that includes herbal and OTC products is essential for safe induction of anesthesia and optimization of outcomes during and following surgery. In general, medications with the potential to induce withdrawal symptoms should be continued. The use of nonessential medications that can increase surgical risk should be discontinued. If neither of these conditions applies, consider the patient’s risk profile and the risk of the procedure when making perioperative management decisions. Be mindful of withdrawal syndromes and resume medications with the potential for such syndromes as soon as possible.
DISCUSSION
Comment from the audience: In regard to your comment that diuretics are typically held on the morning of surgery, my institution recently completed a randomized placebo-controlled trial (publication is pending) in which we studied the effect of continuing or not continuing furosemide preoperatively. We found no difference in the occurrence of intraoperative hypotension between the two groups. It will be interesting to see if these findings change practice over time.
Dr. Whinney: It’s good to know that hypotension is not a concern with furosemide, but the issue here is not just blood pressure but electrolyte abnormalities that could predispose to arrhythmias. The patients who concern me are those who haven’t been seen by a physician for a while and may be on high doses of furosemide. I would scrutinize such patients closely.
Question from the audience: We see a number of patients on methotrexate and other disease-modifying rheumatologic drugs. Can you comment on the perioperative management of these medications?
Dr. Whinney: Methotrexate has caused some anxiety over the risk of infection, but the literature does not support such concern.24 In fact, it appears that continuing methotrexate is probably advisable because the risk of decompensation of the disease may be worse than the potential infectious risks. The only caveat is the patient with renal insufficiency, in whom the recommendation is to withhold methotrexate for 2 weeks before surgery. While most rheumatologists favor withholding disease-modifying drugs perioperatively, a recent systematic review showed no increased risk of either total or infectious complications with use of immunomodulators including infliximab, azathioprine, and cyclosporine.25 It is still reasonable and prudent to discuss this issue with the patient’s rheumatologist. Hydroxychloroquine is safe to continue.
Comment from the audience: First, I would like to urge everyone to be mindful of medication-related indications for preoperative testing. There are many psychotropic drugs that prolong the QT interval and thus constitute an indication for a baseline electrocardiogram prior to surgery. Second, I believe there is a mythology in the perioperative community about the bleeding risk associated with omega-3 fatty acids and vitamin E. Can you comment on the bleeding risks associated with each?
Dr. Whinney: There are few data; the fear is based purely on the potential of these compounds to cause bleeding. Neither is beneficial for short-term quality of life or for chronic prevention, and there’s no withdrawal syndrome from either. So I generally withhold them, but if the patient is still taking them up to the day of surgery, it doesn’t merit postponing surgery. I generally let the surgeon or the nurse know, and it tends not to be a big deal.
Question from the audience: Do you stop herbal teas, energy drinks, and diet medications such as phentermine prior to surgery?
Dr. Whinney: You need to know which diet medications the patient is taking. The problem with many of the OTC products is that they may or may not be considered drugs, so they may not be approved by the FDA and thus you don’t know what the patient is actually taking. For the most part, a diet medication does not contribute to short-term quality of life. My aim is to get the patient through surgery as safely as possible, so if a patient is taking an agent with ingredients, known or unknown, with an interaction potential, then I will stop it.
The two types of diet agents are those that block the absorption of fat, which could interact with other oral agents given at the same time, and those that act via the gastrointestinal tract. I generally withhold the fat-absorption blockers the day before surgery. Phentermine has the potential for catecholinergic reactions or sympathomimetic actions. I would put it in the category of herbal-type medicines and withhold it for at least 7 days.
Question from the audience: Can you comment on combination drugs such as losartan/hydrochlorothiazide on the morning of surgery?
Dr. Whinney: The ARB losartan may have more physiologic benefit than the diuretic, so I would prescribe a single dose of losartan the morning of surgery if I had decided to continue this class of medication for uncontrolled hypertension or concern over heart failure decompensation. The same is true for a beta-blocker/diuretic combination product; I will prescribe the beta-blocker component individually and tell the patient to take it the morning of surgery.
Question from the audience: I’m confused by the recommendation to stop hydrochlorothiazide. It’s a far less potent diuretic than furosemide. Does the risk of stopping it, with resulting blood pressure elevation, outweigh the risk of a mild hypotensive response because of a mild diuretic effect? I’m aware of no data on the risk of stopping hydrochlorothiazide—are you?
Dr. Whinney: There are no data. Again, the recommendation is based on the physiology of the drug, as well as on expert consensus and opinion. Since anesthesia has a vasodilatory effect with a hypotensive response, it’s probably reasonable to hold hydrochlorothiazide if its only indication is for hypertension. That’s the logic behind the recommendation. If you continue it the day of surgery, it may not necessarily hurt, but we’re not certain.
Question from the audience: The implication from your third case study was that alendronate should be held. What’s the basis of that recommendation?
Dr. Whinney: First, the patient has to be upright for 30 minutes after taking alendronate, which could be a problem on the morning of surgery. Also, withholding it will not impair short-term quality of life; it’s a weekly medication, so the patient can take her next dose once she’s up and ambulatory.
Question from the audience: What do you for young women on oral contraceptives? I’m lucky if I see them within 7 days of surgery.
Dr. Whinney: You’re bringing up the concern with exogenous hormones and the risk of venous thromboembolism (VTE), a risk that clearly is increased with the hypercoagulable milieu of surgery. The recommendation is to stop hormone therapy 30 to 45 days prior to surgery in these patients. As you note, however, we don’t get the chance to see patients during that window of opportunity. So the question is whether stopping hormones within a shorter time period results in an incremental benefit. And that is not necessarily the case. These patients should be seen as being at risk for VTE and be given appropriate VTE prophylaxis. In fact, in the similar context of menopausal hormone therapy, a study among women undergoing orthopedic surgery showed that as long as they received appropriate VTE prophylaxis, there was no significant difference in VTE rates between the women whose hormone therapy was withheld versus those who continued it.26
Question from the audience: Are there concerns about withdrawal in patients with peripheral vascular disease treated with cilostazol or pentoxifylline?
Dr. Whinney: It’s not particularly well studied. Guidelines from the American College of Physicians suggest to hold these agents for elective surgeries.27 With respect to antiplatelet therapies, O’Riordan et al did a systematic review of 99 articles pertaining to antiplatelet agents in the perioperative period and concluded that aspirin should not be stopped in patients going for surgery.28 In vascular surgery, antiplatelet agents may help promote graft patency.
- National patient safety goals. The Joint Commission Web site. http://www.jointcommission.org/patientsafety/nationalpatientsafetygoals/. Accessed July 29, 2009.
- Papadopoulos S, Cook AM. You can withdraw from that? The effects of abrupt discontinuation of medications. Orthopedics 2006; 29:413–417.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg 2008; 143:1222–1226.
- Kennedy JM, van Rij AM, Spears GF, Pettigrew RA, Tucker IG. Polypharmacy in a general surgical unit and consequences of drug withdrawal. Br J Clin Pharmacol 2000; 49:353–362.
- Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med 2006; 166:2525–2531.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm 2004; 61:899–912.
- Muluk V, Macpherson DS. Perioperative medication management. In: Rose BD, ed. UpToDate. Waltham, MA; 2008.
- Connelly CS, Panush RS. Should nonsteroidal anti-inflammatory drugs be stopped before elective surgery? Arch Intern Med 1991; 151:1963–1966.
- Robinson CM, Christie J, Malcolm-Smith N. Nonsteroidal antiinflammatory drugs, perioperative blood loss, and transfusion requirements in elective hip arthroplasty. J Arthroplasty 1993; 8:607–610.
- Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief communication: duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med 2005; 142:506–509.
- González-Correa JA, Arrebola MM, Martín-Salido E, Muñoz-Marin J, de la Cuesta FS, De La Cruz JP. Effects of dexibuprofen on platelet function in humans: comparison with low-dose aspirin. Anesthesiology 2007; 106:218–225.
- Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology 1994; 81:299–307.
- Groban L, Butterworth J. Perioperative management of chronic heart failure. Anesth Analg 2006; 103:557–575.
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
- Brabant SM, Bertrand M, Eyraud D, Darmon PL, Coriat P. The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists. Anesth Analg 1999; 89:1388–1392.
- Feringa HH, Bax JJ, Schouten O, Poldermans D. Protecting the heart with cardiac medication in patients with left ventricular dysfunction undergoing major noncardiac vascular surgery. Semin Cardiothorac Vasc Anesth 2006; 10:25–31.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA 2001; 286:208–216.
- Cui J, Garle M, Eneroth P, Björkhem I. What do commercial ginseng preparations contain? Lancet 1994; 344:134.
- Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding: confusion or confounding? Am J Med 2006; 119:719–727.
- de Abajo FJ, Montero D, Rodríguez LA, Madurga M. Antidepressants and risk of upper gastrointestinal bleeding. Basic Clin Pharmacol Toxicol 2006; 98:304–310.
- Serebruany VL. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med 2006; 119:113–116.
- Stack CG, Rogers P, Linter SP. Monoamine oxidase inhibitors and anaesthesia: a review. Br J Anaesth 1988; 60:222–227.
- Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis 2001; 60:214–217.
- Subramanian V, Pollok RC, Kang JY, Kumar D. Systematic review of postoperative complications in patients with inflammatory bowel disease treated with immunomodulators. Br J Surg 2006; 93:793–799.
- Hurbanek JG, Jaffer AK, Morra N, Karafa M, Brotman DJ. Postmenopausal hormone replacement and venous thromboembolism following hip and knee arthroplasty. Thromb Haemost 2004; 92:337–343.
- Cohn SL. Perioperative medication management. American College of Physicians’ PIER (Physicians’ Information and Education Resource) Web site. http://pier.acponline.org/physicians/diseases/d835/diagnosis/d835-s3.html. Posted May 29, 2009. Accessed August 14, 2009.
- O’Riordan JM, Margey RJ, Blake G, O’Connell R. Antiplatelet agents in the perioperative period. Arch Surg 2009; 144:69–76.
As a hospitalist who practices in a perioperative clinic, I probably spend more of my time with patients reviewing and discussing the medications they are taking than on any other single subject. Surgical patients—many of whom are elderly—commonly are on multiple medications, have renal or hepatic disease that can alter drug metabolism, and may not be adequately educated about their medication regimens.
Patient safety is the overriding concern behind perioperative medication management, consistent with the medication-related objectives in the Joint Commission’s 2009 National Patient Safety Goals.1 The increasing surgical burden that comes with an aging population, along with rising expectations for functional recovery, has likewise elevated the importance of perioperative medication management.
Despite these demands, there is scant evidence from randomized controlled trials to directly guide perioperative medication management. For this reason, recommendations in this area rely largely on other forms of evidence, including expert consensus, case reports, in vitro studies, recommendations from pharmaceutical companies, and other known data (pharmacokinetics, drug interactions with anesthetic agents, and effects of the agent on the primary disease and on perioperative risk).
This article reviews general principles of perioperative medication management and then presents four case vignettes to explore perioperative recommendations for a number of common medication classes. It is not intended as a comprehensive review of the perioperative management of all medications, as numerous classes (antiplatelets, beta-blockers, oral hypogycemic agents, insulin, statins) are discussed in detail elsewhere in this proceedings supplement.
GENERAL CONSIDERATIONS IN MEDICATION MANAGEMENT
A comprehensive medication history is fundamental
Effective perioperative management of medications requires an understanding of the patient and his or her comorbidities so that the risk of perioperative decompensation can be gauged. This understanding stems from a thorough medical history that includes a comprehensive medication history to provide a complete inventory of the following:
- All prescription medications
- All over-the-counter (OTC) agents (including nonsteroidal anti-inflammatory drugs [NSAIDs])
- All vitamins
- All herbal medications.
When to stop, when to resume?
Guidance on stopping and resuming medications in the perioperative period is relatively absent from the literature. General considerations include the following:
- The potential for withdrawal when stopping a medication
- The progression of disease with interruption of drug therapy
- The potential for interactions with anesthetic agents if the medication is continued.
Withdrawal potential
Abrupt discontinuation of some drugs may lead to unnecessary complications due to the potential for withdrawal. Common medications that have been associated with withdrawal symptoms are selective serotonin reuptake inhibitors (SSRIs), beta-blockers, clonidine, statins, and corticosteroids.2 A recent systematic literature review concluded that continuation of chronic corticosteroid therapy without supplemental (stress) doses of corticosteroids is appropriate unless patients have primary disease of the hypothalamic-pituitary-adrenal axis, in which case perioperative stress dosing is recommended to avoid acute adrenal insufficiency (addisonian crisis).3
Patients on chronic drugs are more likely to have complications
In a medication survey of 1,025 patients admitted to a general surgery unit, Kennedy et al reported that 49% of the patients were taking medications (other than vitamins) unrelated to their surgical procedure.4 Even while this percentage is considerably lower than what I observe in my practice, this study showed that medication use has important perioperative consequences4:
- The odds ratio for a postoperative complication was 2.7 (95% CI, 1.76–4.04) if patients were taking a drug unrelated to their surgery.
- The risk of a complication was particularly elevated if patients were taking cardiovascular drugs or agents that act on the central nervous system; if patients were on NPO (“nothing by mouth”) orders for more than 24 hours before surgery; and if the operation was more than 1 hour in duration. These findings could reflect destabilization of the disease processes for which the patients were taking chronic medications that required interruption.
Unintended discontinuation of chronic drugs
Stopping a chronic medication for a surgical procedure raises the possibility that its resumption could be overlooked, especially since medical errors are particularly common in the transition between health care settings following hospital discharge. A population-based cohort study among all elderly patients discharged from Ontario, Canada, hospitals over a 5½-year period found that 11.4% of patients undergoing elective surgery did not resume their indicated chronic warfarin therapy within 6 months after its presurgical discontinuation.5 Although 6-month rates of unintended failure to resume therapy were lower for statins (4%) and ophthalmic beta-blocker drops (8%),5 these findings underscore that drug discontinuation always carries a risk that therapy might not be resumed as indicated.
Additional considerations
Stress response to surgery. Decisions about perioperative drug therapy should always take into account the stress response to surgery and the challenge it presents to homeostasis in the face of increased sympathetic tone and release of pituitary hormones.
Unreliable absorption of oral medications. Surgery and the postoperative state can lead to unreliable absorption of oral drugs for any of a number of reasons: villous atrophy, diminished blood flow to the gut, edema, mucosal ischemia, diminished motility from postoperative ileus, and use of narcotics.6
Take-away general principles
The following principles can be applied to guide perioperative medication management in a general sense7:
- Continue medications with withdrawal potential
- Discontinue medications that increase surgical risk and are not essential for short-term quality of life
- Use clinical judgment when neither of the above two principles applies, but be mindful that many other medications are given in the narrow perioperative time window and that metabolism and elimination of chronic drugs may be altered.
CASE 1: A PATIENT ON A NONPRESCRIPTION NSAID FOR SEVERE ARTHRITIS
A 55-year-old man with severe osteoarthritis is scheduled for total hip arthroplasy in 2 days. He stopped his aspirin (325 mg/day) 1 week ago but continued taking ibuprofen 600 three times daily with food, explaining that “no one told me to stop.” His last dose was yesterday evening.
Question: What should you do?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop the ibuprofen now
C. Check his bleeding time and proceed if normal
D. Just tell the patient to stop the ibuprofen now
E. Proceed to the operating room regardless of the ibuprofen dose
The best approach would be to notify the surgeon and tell the patient to stop the ibuprofen now. NSAIDs such as ibuprofen reversibly inhibit platelet cyclooxygenase (COX), diminish thromboxane A2 production, diminish platelet aggregation, and can increase bleeding time measurement and overall bleeding risk. They can induce renal failure in combination with other drugs, especially in the setting of hypotension.8,9 COX-2 inhibitors have less effect on platelet function but retain the potential for renal toxicity and also confer well-known cardiovascular risks.
In the past, NSAIDs were typically held for 7 days before surgery, but this practice was not supported with much evidence. In vitro assessment indicates that platelet function normalizes within 24 hours after cessation of regular ibuprofen or dexibuprofen in healthy individuals.10,11
Since NSAIDs vary in their effect on bleeding time, which does not correlate well with elimination half-life, a general recommendation is to stop most NSAIDs at least 3 days before surgery.
CASE 2: A PATIENT ON MULTIPLE CARDIOVASCULAR DRUGS
A 67-year-old man with dilated cardiomyopathy and an ejection fraction of 25% (well compensated) is scheduled for a laparoscopic cholecystectomy tomorrow. He is taking lisinopril (40 mg/day), irbesartan (150 mg/day), and furosemide (80 mg/day).
Question: What is your advice?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop his medications now
C. Hold all of the above medications on the morning of surgery
D. Proceed to the operating room with the usual doses of his medications on the morning of surgery
The best approach is to withhold these medications on the morning of surgery.
Diuretics are typically held on the morning of surgery because of the potential for hypovolemia and electrolyte depletion.
Angiotensin-converting enzyme (ACE) inhibitors intensify the hypotensive effects of anesthesia induction. Because angiotensin II plays a key role in maintaining circulating volume in response to stressors, volume deficits can occur in ACE inhibitor-treated patients as angiotensin II cannot compensate for venous pooling of blood, resulting in diminished cardiac output and arterial hypotension. However, continued renin-angiotensin system suppression may protect regional circulation, as has been demonstrated by reduced release of cardiac enzymes with ACE inhibitor continuation (compared with interruption) in cardiac surgery patients. ACE inhibitors also have a renal protective effect, preserving glomerular filtration rate in patients undergoing aortic abdominal aneurysm repair or coronary artery bypass graft surgery. Hypotension with ACE inhibition is treatable with sympathomimetics, alpha-agonists, and intravenous fluids.12–15
If a patient’s ACE inhibitor is stopped, be prepared for rebound postoperative hypertension. The probability of postoperative atrial fibrillation is also increased with ACE inhibitor interruption.14 In patients with left ventricular dysfunction undergoing noncardiac vascular surgery, continued ACE inhibition is associated with reduced mortality.16 These data argue, at the very least, for prompt resumption of ACE inhibitors after surgery.
Angiotensin receptor blockers (ARBs) have largely the same clinical benefits as do ACE inhibitors. These agents also increase the risk of hypotension upon induction of anesthesia, and this hypotension is not as responsive to conventional vasopressors such as ephedrine and phenylephrine; a better response is achieved with vasopressin.15 In light of the long half-life of ARBs, current thinking is to withhold them 24 hours before surgery.
Rosenman et al recently published a meta-analysis of five studies assessing the effects of continuing or withholding ACE inhibitors and ARBs in the preoperative period.17 They found a statistically significant increase in the incidence of perioperative hypotension in patients in whom the drugs were continued compared with those in whom the drugs were withheld (relative risk = 1.50; 95% CI, 1.15–1.96), but there was no significant difference in the rate of perioperative MI between the two groups. Notably, the indication for ACE inhibitor or ARB use in all of the studies was hypertension, not heart failure.
My approach to the perioperative management of ACE inhibitors and ARBs is to withhold them on the morning of surgery (in the case of ARBs, 24 hours prior to surgery) if their only indication is for hypertension and if the patient’s blood pressure is well controlled. If the patient has another indication for these agents or has hypertension that is not well controlled, I am inclined to continue these agents but will first discuss the decision with the anesthesiologist.
CASE 3: A PATIENT TAKING HERBAL MEDICATIONS
A 68-year-old woman with a history of hypertension, osteoarthritis, and osteoporosis is scheduled for total hip replacement in 7 days. Her medications include atenolol, hydrochlorothiazide, and alendronate. She also reports taking some natural herbal medications. She does not recall their names initially but calls back with the names: ginkgo biloba for her memory and echinacea for her immune system.
Question: What are your recommendations?
A. Stop all medications now except atenolol and proceed to surgery
B. Stop the herbals now but take all other medications on the morning of surgery
C. Stop the herbals now and take only atenolol on the morning of surgery
D. Continue all medications now and take atenolol and the herbals on the morning of surgery
E. Cancel the surgery and call an herbalist for guidance
The best strategy is to stop the herbals now and tell her to take only atenolol (a beta-blocker) on the morning of surgery.
Because the US Food and Drug Administration (FDA) does not regulate herbal products, the contents of these products can vary widely. For example, an analysis using mass spectrometry of 50 commercial ginseng products from 11 countries found that the ginseng content varied from 0% (six preparations) to 9%.19 Catecholamine-type compounds were found in some of the products.19
Because of the uncertainty over their actual contents, herbal medications should be stopped at least 7 days prior to surgery. If a patient is still taking herbal supplements on the day before surgery, I typically alert the anesthesiologist and surgeon.
CASE 4: A PATIENT ON MULTIPLE PSYCHOTROPICS
A 38-year-old woman with a history of severe major depression is scheduled for a mastectomy for breast cancer the next day. Her medications include fluoxetine, lorazepam, and phenelzine, all of which she has been taking for many years.
Question: What is your course of action?
A. Call the surgeon and cancel the surgery
B. Call the surgeon and notify the day-of-surgery anesthesiologist that the patient is taking these agents
C. Stop all the medications now and proceed to the operating room
D. Request a psychiatric consult for an alternative drug regimen
E. Proceed and advise the patient to take all of these agents on the morning of surgery
My approach would be to notify the day-of-surgery anesthesiologist, specifically about the phenelzine, which is a monoamine oxidase (MAO) inhibitor (see below). The other two agents can be taken on the morning of surgery, although fluoxetine has a long half-life, so missing a dose should not be problematic, and lorazepam can be given intravenously if needed.
SSRIs, including fluoxetine, are generally safe perioperatively. Serotonin depletion from platelets, however, increases the risk of bleeding, especially gastrointestinal bleeding, when SSRIs are used with NSAIDs.20–22 A neurosurgical procedure may therefore be especially risky in patients who have not stopped their SSRI if they are also taking an NSAID or an herbal medication that may increase the risk of bleeding. The caveat to stopping SSRIs is the potential for a minor withdrawal syndrome.
Tricyclic antidepressants inhibit the reuptake of norepinephrine and serotonin and may increase the action of sympathomimetics. Although arrhythmias are thought to be a concern with tricyclics, there are no reported cases of association in the literature. In general, I advise continuing triclyclics perioperatively, especially in patients who are on high doses.
Benzodiazepines, including lorazepam, are safe to use perioperatively, and a potential for withdrawal symptoms (hypertension, agitation, delirium, seizures) argues against their discontinuation. Chronic benzodiazepine use may increase anesthetic requirements.
Antipsychotic agents, which include haloperidol, olanzapine, risperidone, and ziprasidone, have multiple routes of administration—intramuscular, oral, sublingual, and intravenous. These agents are generally safe to use in the perioperative period.
MAO inhibitors, including phenelzine, are no longer commonly used and are typically reserved for the treatment of refractory depression. But they merit attention, as their use can cause accumulation of biogenic amines in the central and autonomic nervous systems. There are two types of MAO reactions—excitatory and depressive. Excitatory reactions lead to serotonin syndrome. Depressive reactions induce inhibition of hepatic microsomal enzymes, leading to narcotic accumulation and increased sedation.23
MAO inhibitors are also of concern because of their many drug interactions. When used with indirect sympathomimetics such as ephedrine, they promote a massive release of stored norepinephrine, leading to severe hypertension. When used with opioids like meperidine and dextromethorphan, MAO inhibitors are associated with a serotonin syndrome characterized by agitation, headache, fever, seizures, coma, and death.
Discontinuing MAO inhibitors before the day of surgery is no longer universally recommended, due to the risk of precipitating an exacerbation of major depression. Safe anesthetic regimens in the setting of MAO inhibitors involve avoidance of meperidine (morphine and fentanyl are safe) and use of only direct-acting sympathomimetics.
CONCLUSIONS
A good medication history that includes herbal and OTC products is essential for safe induction of anesthesia and optimization of outcomes during and following surgery. In general, medications with the potential to induce withdrawal symptoms should be continued. The use of nonessential medications that can increase surgical risk should be discontinued. If neither of these conditions applies, consider the patient’s risk profile and the risk of the procedure when making perioperative management decisions. Be mindful of withdrawal syndromes and resume medications with the potential for such syndromes as soon as possible.
DISCUSSION
Comment from the audience: In regard to your comment that diuretics are typically held on the morning of surgery, my institution recently completed a randomized placebo-controlled trial (publication is pending) in which we studied the effect of continuing or not continuing furosemide preoperatively. We found no difference in the occurrence of intraoperative hypotension between the two groups. It will be interesting to see if these findings change practice over time.
Dr. Whinney: It’s good to know that hypotension is not a concern with furosemide, but the issue here is not just blood pressure but electrolyte abnormalities that could predispose to arrhythmias. The patients who concern me are those who haven’t been seen by a physician for a while and may be on high doses of furosemide. I would scrutinize such patients closely.
Question from the audience: We see a number of patients on methotrexate and other disease-modifying rheumatologic drugs. Can you comment on the perioperative management of these medications?
Dr. Whinney: Methotrexate has caused some anxiety over the risk of infection, but the literature does not support such concern.24 In fact, it appears that continuing methotrexate is probably advisable because the risk of decompensation of the disease may be worse than the potential infectious risks. The only caveat is the patient with renal insufficiency, in whom the recommendation is to withhold methotrexate for 2 weeks before surgery. While most rheumatologists favor withholding disease-modifying drugs perioperatively, a recent systematic review showed no increased risk of either total or infectious complications with use of immunomodulators including infliximab, azathioprine, and cyclosporine.25 It is still reasonable and prudent to discuss this issue with the patient’s rheumatologist. Hydroxychloroquine is safe to continue.
Comment from the audience: First, I would like to urge everyone to be mindful of medication-related indications for preoperative testing. There are many psychotropic drugs that prolong the QT interval and thus constitute an indication for a baseline electrocardiogram prior to surgery. Second, I believe there is a mythology in the perioperative community about the bleeding risk associated with omega-3 fatty acids and vitamin E. Can you comment on the bleeding risks associated with each?
Dr. Whinney: There are few data; the fear is based purely on the potential of these compounds to cause bleeding. Neither is beneficial for short-term quality of life or for chronic prevention, and there’s no withdrawal syndrome from either. So I generally withhold them, but if the patient is still taking them up to the day of surgery, it doesn’t merit postponing surgery. I generally let the surgeon or the nurse know, and it tends not to be a big deal.
Question from the audience: Do you stop herbal teas, energy drinks, and diet medications such as phentermine prior to surgery?
Dr. Whinney: You need to know which diet medications the patient is taking. The problem with many of the OTC products is that they may or may not be considered drugs, so they may not be approved by the FDA and thus you don’t know what the patient is actually taking. For the most part, a diet medication does not contribute to short-term quality of life. My aim is to get the patient through surgery as safely as possible, so if a patient is taking an agent with ingredients, known or unknown, with an interaction potential, then I will stop it.
The two types of diet agents are those that block the absorption of fat, which could interact with other oral agents given at the same time, and those that act via the gastrointestinal tract. I generally withhold the fat-absorption blockers the day before surgery. Phentermine has the potential for catecholinergic reactions or sympathomimetic actions. I would put it in the category of herbal-type medicines and withhold it for at least 7 days.
Question from the audience: Can you comment on combination drugs such as losartan/hydrochlorothiazide on the morning of surgery?
Dr. Whinney: The ARB losartan may have more physiologic benefit than the diuretic, so I would prescribe a single dose of losartan the morning of surgery if I had decided to continue this class of medication for uncontrolled hypertension or concern over heart failure decompensation. The same is true for a beta-blocker/diuretic combination product; I will prescribe the beta-blocker component individually and tell the patient to take it the morning of surgery.
Question from the audience: I’m confused by the recommendation to stop hydrochlorothiazide. It’s a far less potent diuretic than furosemide. Does the risk of stopping it, with resulting blood pressure elevation, outweigh the risk of a mild hypotensive response because of a mild diuretic effect? I’m aware of no data on the risk of stopping hydrochlorothiazide—are you?
Dr. Whinney: There are no data. Again, the recommendation is based on the physiology of the drug, as well as on expert consensus and opinion. Since anesthesia has a vasodilatory effect with a hypotensive response, it’s probably reasonable to hold hydrochlorothiazide if its only indication is for hypertension. That’s the logic behind the recommendation. If you continue it the day of surgery, it may not necessarily hurt, but we’re not certain.
Question from the audience: The implication from your third case study was that alendronate should be held. What’s the basis of that recommendation?
Dr. Whinney: First, the patient has to be upright for 30 minutes after taking alendronate, which could be a problem on the morning of surgery. Also, withholding it will not impair short-term quality of life; it’s a weekly medication, so the patient can take her next dose once she’s up and ambulatory.
Question from the audience: What do you for young women on oral contraceptives? I’m lucky if I see them within 7 days of surgery.
Dr. Whinney: You’re bringing up the concern with exogenous hormones and the risk of venous thromboembolism (VTE), a risk that clearly is increased with the hypercoagulable milieu of surgery. The recommendation is to stop hormone therapy 30 to 45 days prior to surgery in these patients. As you note, however, we don’t get the chance to see patients during that window of opportunity. So the question is whether stopping hormones within a shorter time period results in an incremental benefit. And that is not necessarily the case. These patients should be seen as being at risk for VTE and be given appropriate VTE prophylaxis. In fact, in the similar context of menopausal hormone therapy, a study among women undergoing orthopedic surgery showed that as long as they received appropriate VTE prophylaxis, there was no significant difference in VTE rates between the women whose hormone therapy was withheld versus those who continued it.26
Question from the audience: Are there concerns about withdrawal in patients with peripheral vascular disease treated with cilostazol or pentoxifylline?
Dr. Whinney: It’s not particularly well studied. Guidelines from the American College of Physicians suggest to hold these agents for elective surgeries.27 With respect to antiplatelet therapies, O’Riordan et al did a systematic review of 99 articles pertaining to antiplatelet agents in the perioperative period and concluded that aspirin should not be stopped in patients going for surgery.28 In vascular surgery, antiplatelet agents may help promote graft patency.
As a hospitalist who practices in a perioperative clinic, I probably spend more of my time with patients reviewing and discussing the medications they are taking than on any other single subject. Surgical patients—many of whom are elderly—commonly are on multiple medications, have renal or hepatic disease that can alter drug metabolism, and may not be adequately educated about their medication regimens.
Patient safety is the overriding concern behind perioperative medication management, consistent with the medication-related objectives in the Joint Commission’s 2009 National Patient Safety Goals.1 The increasing surgical burden that comes with an aging population, along with rising expectations for functional recovery, has likewise elevated the importance of perioperative medication management.
Despite these demands, there is scant evidence from randomized controlled trials to directly guide perioperative medication management. For this reason, recommendations in this area rely largely on other forms of evidence, including expert consensus, case reports, in vitro studies, recommendations from pharmaceutical companies, and other known data (pharmacokinetics, drug interactions with anesthetic agents, and effects of the agent on the primary disease and on perioperative risk).
This article reviews general principles of perioperative medication management and then presents four case vignettes to explore perioperative recommendations for a number of common medication classes. It is not intended as a comprehensive review of the perioperative management of all medications, as numerous classes (antiplatelets, beta-blockers, oral hypogycemic agents, insulin, statins) are discussed in detail elsewhere in this proceedings supplement.
GENERAL CONSIDERATIONS IN MEDICATION MANAGEMENT
A comprehensive medication history is fundamental
Effective perioperative management of medications requires an understanding of the patient and his or her comorbidities so that the risk of perioperative decompensation can be gauged. This understanding stems from a thorough medical history that includes a comprehensive medication history to provide a complete inventory of the following:
- All prescription medications
- All over-the-counter (OTC) agents (including nonsteroidal anti-inflammatory drugs [NSAIDs])
- All vitamins
- All herbal medications.
When to stop, when to resume?
Guidance on stopping and resuming medications in the perioperative period is relatively absent from the literature. General considerations include the following:
- The potential for withdrawal when stopping a medication
- The progression of disease with interruption of drug therapy
- The potential for interactions with anesthetic agents if the medication is continued.
Withdrawal potential
Abrupt discontinuation of some drugs may lead to unnecessary complications due to the potential for withdrawal. Common medications that have been associated with withdrawal symptoms are selective serotonin reuptake inhibitors (SSRIs), beta-blockers, clonidine, statins, and corticosteroids.2 A recent systematic literature review concluded that continuation of chronic corticosteroid therapy without supplemental (stress) doses of corticosteroids is appropriate unless patients have primary disease of the hypothalamic-pituitary-adrenal axis, in which case perioperative stress dosing is recommended to avoid acute adrenal insufficiency (addisonian crisis).3
Patients on chronic drugs are more likely to have complications
In a medication survey of 1,025 patients admitted to a general surgery unit, Kennedy et al reported that 49% of the patients were taking medications (other than vitamins) unrelated to their surgical procedure.4 Even while this percentage is considerably lower than what I observe in my practice, this study showed that medication use has important perioperative consequences4:
- The odds ratio for a postoperative complication was 2.7 (95% CI, 1.76–4.04) if patients were taking a drug unrelated to their surgery.
- The risk of a complication was particularly elevated if patients were taking cardiovascular drugs or agents that act on the central nervous system; if patients were on NPO (“nothing by mouth”) orders for more than 24 hours before surgery; and if the operation was more than 1 hour in duration. These findings could reflect destabilization of the disease processes for which the patients were taking chronic medications that required interruption.
Unintended discontinuation of chronic drugs
Stopping a chronic medication for a surgical procedure raises the possibility that its resumption could be overlooked, especially since medical errors are particularly common in the transition between health care settings following hospital discharge. A population-based cohort study among all elderly patients discharged from Ontario, Canada, hospitals over a 5½-year period found that 11.4% of patients undergoing elective surgery did not resume their indicated chronic warfarin therapy within 6 months after its presurgical discontinuation.5 Although 6-month rates of unintended failure to resume therapy were lower for statins (4%) and ophthalmic beta-blocker drops (8%),5 these findings underscore that drug discontinuation always carries a risk that therapy might not be resumed as indicated.
Additional considerations
Stress response to surgery. Decisions about perioperative drug therapy should always take into account the stress response to surgery and the challenge it presents to homeostasis in the face of increased sympathetic tone and release of pituitary hormones.
Unreliable absorption of oral medications. Surgery and the postoperative state can lead to unreliable absorption of oral drugs for any of a number of reasons: villous atrophy, diminished blood flow to the gut, edema, mucosal ischemia, diminished motility from postoperative ileus, and use of narcotics.6
Take-away general principles
The following principles can be applied to guide perioperative medication management in a general sense7:
- Continue medications with withdrawal potential
- Discontinue medications that increase surgical risk and are not essential for short-term quality of life
- Use clinical judgment when neither of the above two principles applies, but be mindful that many other medications are given in the narrow perioperative time window and that metabolism and elimination of chronic drugs may be altered.
CASE 1: A PATIENT ON A NONPRESCRIPTION NSAID FOR SEVERE ARTHRITIS
A 55-year-old man with severe osteoarthritis is scheduled for total hip arthroplasy in 2 days. He stopped his aspirin (325 mg/day) 1 week ago but continued taking ibuprofen 600 three times daily with food, explaining that “no one told me to stop.” His last dose was yesterday evening.
Question: What should you do?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop the ibuprofen now
C. Check his bleeding time and proceed if normal
D. Just tell the patient to stop the ibuprofen now
E. Proceed to the operating room regardless of the ibuprofen dose
The best approach would be to notify the surgeon and tell the patient to stop the ibuprofen now. NSAIDs such as ibuprofen reversibly inhibit platelet cyclooxygenase (COX), diminish thromboxane A2 production, diminish platelet aggregation, and can increase bleeding time measurement and overall bleeding risk. They can induce renal failure in combination with other drugs, especially in the setting of hypotension.8,9 COX-2 inhibitors have less effect on platelet function but retain the potential for renal toxicity and also confer well-known cardiovascular risks.
In the past, NSAIDs were typically held for 7 days before surgery, but this practice was not supported with much evidence. In vitro assessment indicates that platelet function normalizes within 24 hours after cessation of regular ibuprofen or dexibuprofen in healthy individuals.10,11
Since NSAIDs vary in their effect on bleeding time, which does not correlate well with elimination half-life, a general recommendation is to stop most NSAIDs at least 3 days before surgery.
CASE 2: A PATIENT ON MULTIPLE CARDIOVASCULAR DRUGS
A 67-year-old man with dilated cardiomyopathy and an ejection fraction of 25% (well compensated) is scheduled for a laparoscopic cholecystectomy tomorrow. He is taking lisinopril (40 mg/day), irbesartan (150 mg/day), and furosemide (80 mg/day).
Question: What is your advice?
A. Call the surgeon and cancel the surgery
B. Call the surgeon to notify, and tell the patient to stop his medications now
C. Hold all of the above medications on the morning of surgery
D. Proceed to the operating room with the usual doses of his medications on the morning of surgery
The best approach is to withhold these medications on the morning of surgery.
Diuretics are typically held on the morning of surgery because of the potential for hypovolemia and electrolyte depletion.
Angiotensin-converting enzyme (ACE) inhibitors intensify the hypotensive effects of anesthesia induction. Because angiotensin II plays a key role in maintaining circulating volume in response to stressors, volume deficits can occur in ACE inhibitor-treated patients as angiotensin II cannot compensate for venous pooling of blood, resulting in diminished cardiac output and arterial hypotension. However, continued renin-angiotensin system suppression may protect regional circulation, as has been demonstrated by reduced release of cardiac enzymes with ACE inhibitor continuation (compared with interruption) in cardiac surgery patients. ACE inhibitors also have a renal protective effect, preserving glomerular filtration rate in patients undergoing aortic abdominal aneurysm repair or coronary artery bypass graft surgery. Hypotension with ACE inhibition is treatable with sympathomimetics, alpha-agonists, and intravenous fluids.12–15
If a patient’s ACE inhibitor is stopped, be prepared for rebound postoperative hypertension. The probability of postoperative atrial fibrillation is also increased with ACE inhibitor interruption.14 In patients with left ventricular dysfunction undergoing noncardiac vascular surgery, continued ACE inhibition is associated with reduced mortality.16 These data argue, at the very least, for prompt resumption of ACE inhibitors after surgery.
Angiotensin receptor blockers (ARBs) have largely the same clinical benefits as do ACE inhibitors. These agents also increase the risk of hypotension upon induction of anesthesia, and this hypotension is not as responsive to conventional vasopressors such as ephedrine and phenylephrine; a better response is achieved with vasopressin.15 In light of the long half-life of ARBs, current thinking is to withhold them 24 hours before surgery.
Rosenman et al recently published a meta-analysis of five studies assessing the effects of continuing or withholding ACE inhibitors and ARBs in the preoperative period.17 They found a statistically significant increase in the incidence of perioperative hypotension in patients in whom the drugs were continued compared with those in whom the drugs were withheld (relative risk = 1.50; 95% CI, 1.15–1.96), but there was no significant difference in the rate of perioperative MI between the two groups. Notably, the indication for ACE inhibitor or ARB use in all of the studies was hypertension, not heart failure.
My approach to the perioperative management of ACE inhibitors and ARBs is to withhold them on the morning of surgery (in the case of ARBs, 24 hours prior to surgery) if their only indication is for hypertension and if the patient’s blood pressure is well controlled. If the patient has another indication for these agents or has hypertension that is not well controlled, I am inclined to continue these agents but will first discuss the decision with the anesthesiologist.
CASE 3: A PATIENT TAKING HERBAL MEDICATIONS
A 68-year-old woman with a history of hypertension, osteoarthritis, and osteoporosis is scheduled for total hip replacement in 7 days. Her medications include atenolol, hydrochlorothiazide, and alendronate. She also reports taking some natural herbal medications. She does not recall their names initially but calls back with the names: ginkgo biloba for her memory and echinacea for her immune system.
Question: What are your recommendations?
A. Stop all medications now except atenolol and proceed to surgery
B. Stop the herbals now but take all other medications on the morning of surgery
C. Stop the herbals now and take only atenolol on the morning of surgery
D. Continue all medications now and take atenolol and the herbals on the morning of surgery
E. Cancel the surgery and call an herbalist for guidance
The best strategy is to stop the herbals now and tell her to take only atenolol (a beta-blocker) on the morning of surgery.
Because the US Food and Drug Administration (FDA) does not regulate herbal products, the contents of these products can vary widely. For example, an analysis using mass spectrometry of 50 commercial ginseng products from 11 countries found that the ginseng content varied from 0% (six preparations) to 9%.19 Catecholamine-type compounds were found in some of the products.19
Because of the uncertainty over their actual contents, herbal medications should be stopped at least 7 days prior to surgery. If a patient is still taking herbal supplements on the day before surgery, I typically alert the anesthesiologist and surgeon.
CASE 4: A PATIENT ON MULTIPLE PSYCHOTROPICS
A 38-year-old woman with a history of severe major depression is scheduled for a mastectomy for breast cancer the next day. Her medications include fluoxetine, lorazepam, and phenelzine, all of which she has been taking for many years.
Question: What is your course of action?
A. Call the surgeon and cancel the surgery
B. Call the surgeon and notify the day-of-surgery anesthesiologist that the patient is taking these agents
C. Stop all the medications now and proceed to the operating room
D. Request a psychiatric consult for an alternative drug regimen
E. Proceed and advise the patient to take all of these agents on the morning of surgery
My approach would be to notify the day-of-surgery anesthesiologist, specifically about the phenelzine, which is a monoamine oxidase (MAO) inhibitor (see below). The other two agents can be taken on the morning of surgery, although fluoxetine has a long half-life, so missing a dose should not be problematic, and lorazepam can be given intravenously if needed.
SSRIs, including fluoxetine, are generally safe perioperatively. Serotonin depletion from platelets, however, increases the risk of bleeding, especially gastrointestinal bleeding, when SSRIs are used with NSAIDs.20–22 A neurosurgical procedure may therefore be especially risky in patients who have not stopped their SSRI if they are also taking an NSAID or an herbal medication that may increase the risk of bleeding. The caveat to stopping SSRIs is the potential for a minor withdrawal syndrome.
Tricyclic antidepressants inhibit the reuptake of norepinephrine and serotonin and may increase the action of sympathomimetics. Although arrhythmias are thought to be a concern with tricyclics, there are no reported cases of association in the literature. In general, I advise continuing triclyclics perioperatively, especially in patients who are on high doses.
Benzodiazepines, including lorazepam, are safe to use perioperatively, and a potential for withdrawal symptoms (hypertension, agitation, delirium, seizures) argues against their discontinuation. Chronic benzodiazepine use may increase anesthetic requirements.
Antipsychotic agents, which include haloperidol, olanzapine, risperidone, and ziprasidone, have multiple routes of administration—intramuscular, oral, sublingual, and intravenous. These agents are generally safe to use in the perioperative period.
MAO inhibitors, including phenelzine, are no longer commonly used and are typically reserved for the treatment of refractory depression. But they merit attention, as their use can cause accumulation of biogenic amines in the central and autonomic nervous systems. There are two types of MAO reactions—excitatory and depressive. Excitatory reactions lead to serotonin syndrome. Depressive reactions induce inhibition of hepatic microsomal enzymes, leading to narcotic accumulation and increased sedation.23
MAO inhibitors are also of concern because of their many drug interactions. When used with indirect sympathomimetics such as ephedrine, they promote a massive release of stored norepinephrine, leading to severe hypertension. When used with opioids like meperidine and dextromethorphan, MAO inhibitors are associated with a serotonin syndrome characterized by agitation, headache, fever, seizures, coma, and death.
Discontinuing MAO inhibitors before the day of surgery is no longer universally recommended, due to the risk of precipitating an exacerbation of major depression. Safe anesthetic regimens in the setting of MAO inhibitors involve avoidance of meperidine (morphine and fentanyl are safe) and use of only direct-acting sympathomimetics.
CONCLUSIONS
A good medication history that includes herbal and OTC products is essential for safe induction of anesthesia and optimization of outcomes during and following surgery. In general, medications with the potential to induce withdrawal symptoms should be continued. The use of nonessential medications that can increase surgical risk should be discontinued. If neither of these conditions applies, consider the patient’s risk profile and the risk of the procedure when making perioperative management decisions. Be mindful of withdrawal syndromes and resume medications with the potential for such syndromes as soon as possible.
DISCUSSION
Comment from the audience: In regard to your comment that diuretics are typically held on the morning of surgery, my institution recently completed a randomized placebo-controlled trial (publication is pending) in which we studied the effect of continuing or not continuing furosemide preoperatively. We found no difference in the occurrence of intraoperative hypotension between the two groups. It will be interesting to see if these findings change practice over time.
Dr. Whinney: It’s good to know that hypotension is not a concern with furosemide, but the issue here is not just blood pressure but electrolyte abnormalities that could predispose to arrhythmias. The patients who concern me are those who haven’t been seen by a physician for a while and may be on high doses of furosemide. I would scrutinize such patients closely.
Question from the audience: We see a number of patients on methotrexate and other disease-modifying rheumatologic drugs. Can you comment on the perioperative management of these medications?
Dr. Whinney: Methotrexate has caused some anxiety over the risk of infection, but the literature does not support such concern.24 In fact, it appears that continuing methotrexate is probably advisable because the risk of decompensation of the disease may be worse than the potential infectious risks. The only caveat is the patient with renal insufficiency, in whom the recommendation is to withhold methotrexate for 2 weeks before surgery. While most rheumatologists favor withholding disease-modifying drugs perioperatively, a recent systematic review showed no increased risk of either total or infectious complications with use of immunomodulators including infliximab, azathioprine, and cyclosporine.25 It is still reasonable and prudent to discuss this issue with the patient’s rheumatologist. Hydroxychloroquine is safe to continue.
Comment from the audience: First, I would like to urge everyone to be mindful of medication-related indications for preoperative testing. There are many psychotropic drugs that prolong the QT interval and thus constitute an indication for a baseline electrocardiogram prior to surgery. Second, I believe there is a mythology in the perioperative community about the bleeding risk associated with omega-3 fatty acids and vitamin E. Can you comment on the bleeding risks associated with each?
Dr. Whinney: There are few data; the fear is based purely on the potential of these compounds to cause bleeding. Neither is beneficial for short-term quality of life or for chronic prevention, and there’s no withdrawal syndrome from either. So I generally withhold them, but if the patient is still taking them up to the day of surgery, it doesn’t merit postponing surgery. I generally let the surgeon or the nurse know, and it tends not to be a big deal.
Question from the audience: Do you stop herbal teas, energy drinks, and diet medications such as phentermine prior to surgery?
Dr. Whinney: You need to know which diet medications the patient is taking. The problem with many of the OTC products is that they may or may not be considered drugs, so they may not be approved by the FDA and thus you don’t know what the patient is actually taking. For the most part, a diet medication does not contribute to short-term quality of life. My aim is to get the patient through surgery as safely as possible, so if a patient is taking an agent with ingredients, known or unknown, with an interaction potential, then I will stop it.
The two types of diet agents are those that block the absorption of fat, which could interact with other oral agents given at the same time, and those that act via the gastrointestinal tract. I generally withhold the fat-absorption blockers the day before surgery. Phentermine has the potential for catecholinergic reactions or sympathomimetic actions. I would put it in the category of herbal-type medicines and withhold it for at least 7 days.
Question from the audience: Can you comment on combination drugs such as losartan/hydrochlorothiazide on the morning of surgery?
Dr. Whinney: The ARB losartan may have more physiologic benefit than the diuretic, so I would prescribe a single dose of losartan the morning of surgery if I had decided to continue this class of medication for uncontrolled hypertension or concern over heart failure decompensation. The same is true for a beta-blocker/diuretic combination product; I will prescribe the beta-blocker component individually and tell the patient to take it the morning of surgery.
Question from the audience: I’m confused by the recommendation to stop hydrochlorothiazide. It’s a far less potent diuretic than furosemide. Does the risk of stopping it, with resulting blood pressure elevation, outweigh the risk of a mild hypotensive response because of a mild diuretic effect? I’m aware of no data on the risk of stopping hydrochlorothiazide—are you?
Dr. Whinney: There are no data. Again, the recommendation is based on the physiology of the drug, as well as on expert consensus and opinion. Since anesthesia has a vasodilatory effect with a hypotensive response, it’s probably reasonable to hold hydrochlorothiazide if its only indication is for hypertension. That’s the logic behind the recommendation. If you continue it the day of surgery, it may not necessarily hurt, but we’re not certain.
Question from the audience: The implication from your third case study was that alendronate should be held. What’s the basis of that recommendation?
Dr. Whinney: First, the patient has to be upright for 30 minutes after taking alendronate, which could be a problem on the morning of surgery. Also, withholding it will not impair short-term quality of life; it’s a weekly medication, so the patient can take her next dose once she’s up and ambulatory.
Question from the audience: What do you for young women on oral contraceptives? I’m lucky if I see them within 7 days of surgery.
Dr. Whinney: You’re bringing up the concern with exogenous hormones and the risk of venous thromboembolism (VTE), a risk that clearly is increased with the hypercoagulable milieu of surgery. The recommendation is to stop hormone therapy 30 to 45 days prior to surgery in these patients. As you note, however, we don’t get the chance to see patients during that window of opportunity. So the question is whether stopping hormones within a shorter time period results in an incremental benefit. And that is not necessarily the case. These patients should be seen as being at risk for VTE and be given appropriate VTE prophylaxis. In fact, in the similar context of menopausal hormone therapy, a study among women undergoing orthopedic surgery showed that as long as they received appropriate VTE prophylaxis, there was no significant difference in VTE rates between the women whose hormone therapy was withheld versus those who continued it.26
Question from the audience: Are there concerns about withdrawal in patients with peripheral vascular disease treated with cilostazol or pentoxifylline?
Dr. Whinney: It’s not particularly well studied. Guidelines from the American College of Physicians suggest to hold these agents for elective surgeries.27 With respect to antiplatelet therapies, O’Riordan et al did a systematic review of 99 articles pertaining to antiplatelet agents in the perioperative period and concluded that aspirin should not be stopped in patients going for surgery.28 In vascular surgery, antiplatelet agents may help promote graft patency.
- National patient safety goals. The Joint Commission Web site. http://www.jointcommission.org/patientsafety/nationalpatientsafetygoals/. Accessed July 29, 2009.
- Papadopoulos S, Cook AM. You can withdraw from that? The effects of abrupt discontinuation of medications. Orthopedics 2006; 29:413–417.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg 2008; 143:1222–1226.
- Kennedy JM, van Rij AM, Spears GF, Pettigrew RA, Tucker IG. Polypharmacy in a general surgical unit and consequences of drug withdrawal. Br J Clin Pharmacol 2000; 49:353–362.
- Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med 2006; 166:2525–2531.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm 2004; 61:899–912.
- Muluk V, Macpherson DS. Perioperative medication management. In: Rose BD, ed. UpToDate. Waltham, MA; 2008.
- Connelly CS, Panush RS. Should nonsteroidal anti-inflammatory drugs be stopped before elective surgery? Arch Intern Med 1991; 151:1963–1966.
- Robinson CM, Christie J, Malcolm-Smith N. Nonsteroidal antiinflammatory drugs, perioperative blood loss, and transfusion requirements in elective hip arthroplasty. J Arthroplasty 1993; 8:607–610.
- Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief communication: duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med 2005; 142:506–509.
- González-Correa JA, Arrebola MM, Martín-Salido E, Muñoz-Marin J, de la Cuesta FS, De La Cruz JP. Effects of dexibuprofen on platelet function in humans: comparison with low-dose aspirin. Anesthesiology 2007; 106:218–225.
- Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology 1994; 81:299–307.
- Groban L, Butterworth J. Perioperative management of chronic heart failure. Anesth Analg 2006; 103:557–575.
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
- Brabant SM, Bertrand M, Eyraud D, Darmon PL, Coriat P. The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists. Anesth Analg 1999; 89:1388–1392.
- Feringa HH, Bax JJ, Schouten O, Poldermans D. Protecting the heart with cardiac medication in patients with left ventricular dysfunction undergoing major noncardiac vascular surgery. Semin Cardiothorac Vasc Anesth 2006; 10:25–31.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA 2001; 286:208–216.
- Cui J, Garle M, Eneroth P, Björkhem I. What do commercial ginseng preparations contain? Lancet 1994; 344:134.
- Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding: confusion or confounding? Am J Med 2006; 119:719–727.
- de Abajo FJ, Montero D, Rodríguez LA, Madurga M. Antidepressants and risk of upper gastrointestinal bleeding. Basic Clin Pharmacol Toxicol 2006; 98:304–310.
- Serebruany VL. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med 2006; 119:113–116.
- Stack CG, Rogers P, Linter SP. Monoamine oxidase inhibitors and anaesthesia: a review. Br J Anaesth 1988; 60:222–227.
- Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis 2001; 60:214–217.
- Subramanian V, Pollok RC, Kang JY, Kumar D. Systematic review of postoperative complications in patients with inflammatory bowel disease treated with immunomodulators. Br J Surg 2006; 93:793–799.
- Hurbanek JG, Jaffer AK, Morra N, Karafa M, Brotman DJ. Postmenopausal hormone replacement and venous thromboembolism following hip and knee arthroplasty. Thromb Haemost 2004; 92:337–343.
- Cohn SL. Perioperative medication management. American College of Physicians’ PIER (Physicians’ Information and Education Resource) Web site. http://pier.acponline.org/physicians/diseases/d835/diagnosis/d835-s3.html. Posted May 29, 2009. Accessed August 14, 2009.
- O’Riordan JM, Margey RJ, Blake G, O’Connell R. Antiplatelet agents in the perioperative period. Arch Surg 2009; 144:69–76.
- National patient safety goals. The Joint Commission Web site. http://www.jointcommission.org/patientsafety/nationalpatientsafetygoals/. Accessed July 29, 2009.
- Papadopoulos S, Cook AM. You can withdraw from that? The effects of abrupt discontinuation of medications. Orthopedics 2006; 29:413–417.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg 2008; 143:1222–1226.
- Kennedy JM, van Rij AM, Spears GF, Pettigrew RA, Tucker IG. Polypharmacy in a general surgical unit and consequences of drug withdrawal. Br J Clin Pharmacol 2000; 49:353–362.
- Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med 2006; 166:2525–2531.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm 2004; 61:899–912.
- Muluk V, Macpherson DS. Perioperative medication management. In: Rose BD, ed. UpToDate. Waltham, MA; 2008.
- Connelly CS, Panush RS. Should nonsteroidal anti-inflammatory drugs be stopped before elective surgery? Arch Intern Med 1991; 151:1963–1966.
- Robinson CM, Christie J, Malcolm-Smith N. Nonsteroidal antiinflammatory drugs, perioperative blood loss, and transfusion requirements in elective hip arthroplasty. J Arthroplasty 1993; 8:607–610.
- Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief communication: duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med 2005; 142:506–509.
- González-Correa JA, Arrebola MM, Martín-Salido E, Muñoz-Marin J, de la Cuesta FS, De La Cruz JP. Effects of dexibuprofen on platelet function in humans: comparison with low-dose aspirin. Anesthesiology 2007; 106:218–225.
- Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology 1994; 81:299–307.
- Groban L, Butterworth J. Perioperative management of chronic heart failure. Anesth Analg 2006; 103:557–575.
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
- Brabant SM, Bertrand M, Eyraud D, Darmon PL, Coriat P. The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists. Anesth Analg 1999; 89:1388–1392.
- Feringa HH, Bax JJ, Schouten O, Poldermans D. Protecting the heart with cardiac medication in patients with left ventricular dysfunction undergoing major noncardiac vascular surgery. Semin Cardiothorac Vasc Anesth 2006; 10:25–31.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA 2001; 286:208–216.
- Cui J, Garle M, Eneroth P, Björkhem I. What do commercial ginseng preparations contain? Lancet 1994; 344:134.
- Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding: confusion or confounding? Am J Med 2006; 119:719–727.
- de Abajo FJ, Montero D, Rodríguez LA, Madurga M. Antidepressants and risk of upper gastrointestinal bleeding. Basic Clin Pharmacol Toxicol 2006; 98:304–310.
- Serebruany VL. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med 2006; 119:113–116.
- Stack CG, Rogers P, Linter SP. Monoamine oxidase inhibitors and anaesthesia: a review. Br J Anaesth 1988; 60:222–227.
- Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis 2001; 60:214–217.
- Subramanian V, Pollok RC, Kang JY, Kumar D. Systematic review of postoperative complications in patients with inflammatory bowel disease treated with immunomodulators. Br J Surg 2006; 93:793–799.
- Hurbanek JG, Jaffer AK, Morra N, Karafa M, Brotman DJ. Postmenopausal hormone replacement and venous thromboembolism following hip and knee arthroplasty. Thromb Haemost 2004; 92:337–343.
- Cohn SL. Perioperative medication management. American College of Physicians’ PIER (Physicians’ Information and Education Resource) Web site. http://pier.acponline.org/physicians/diseases/d835/diagnosis/d835-s3.html. Posted May 29, 2009. Accessed August 14, 2009.
- O’Riordan JM, Margey RJ, Blake G, O’Connell R. Antiplatelet agents in the perioperative period. Arch Surg 2009; 144:69–76.
KEY POINTS
- Common drugs that have been associated with withdrawal symptoms when discontinued preoperatively include selective serotonin reuptake inhibitors (SSRIs), beta-blockers, clonidine, statins, and corticosteroids.
- In general, most nonsteroidal anti-inflammatory drugs should be stopped at least 3 days before surgery.
- Although ACE inhibitors and angiotensin receptor blockers intensify the hypotensive effects of anesthesia, it may be prudent to continue them perioperatively unless their only indication is for hypertension and the patient’s blood pressure is well controlled.
- Herbal medications should be stopped at least 7 days before surgery, owing to the uncertainly over their actual contents.
- Among psychotropics, SSRIs, tricyclic antidepressants, benzodiazepines, and antipsychotics are generally safe to continue perioperatively.
Surgical Comanagement
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
Does unrecognized diabetes in the preoperative period worsen postoperative outcomes?
Managing perioperative risk in the hip fracture patient
Other Literature of Interest
1. Dexter PR, Perkins SM, Mahany KS, Jones K, McDonald CJ. Inpatient computer-based standing orders vs. physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA. 2004; 292: 2366-71.
Past studies have suggested that most patients admitted with severe pneumococcal infections have been hospitalized in the preceding 5 years, and simply being hospitalized is a potential risk factor for later pneumococcal infection. Likewise, hospitalization provides an opportunity to vaccinate high-risk patients against influenza, and raising pneumococcal and influenza immunization rates is a CMS quality improvement priority. Prior investigations have supported the use of labor-intensive manual standing orders as well as computerized reminders, but this prospective trial was conducted in 1998 and 1999 to assess the effectiveness of a computer-based system to screen for eligible patients and then generate orders to perform pneumonia and influenza vaccinations on inpatients at the time of discharge.
Over 13 months, a total of 3777 inpatients were entered into the study. The hospital computer identified patients eligible for vaccination based on common criteria and randomized them to one of two groups of physician teams. For one group of teams, the computer order-entry system would automatically generate vaccination orders at the time of discharge for vaccine-eligible patients; for the other group of teams, only computer reminders were provided to physicians. The outcome measure was administration of vaccine; long-term outcomes such as incidence of subsequent disease or mortality were not measured.
During the study period, 50% of all hospitalized patients were identified as eligible for influenza vaccination; 22% were eligible for pneumococcal vaccination. In each case, the “standing order” group received vaccine more often (influenza: 42% vs. 30%, p<.001; pneumococcal vaccine: 51% vs. 31%). The numbers were subsequently adjusted to allow for patients who had previously received vaccine, but the impressive differences persisted. Nurses reported reasons for non-administration in 98% of the eligible patients who were not vaccinated; the most common reason was patient refusal. It is not clear if the physicians knew that a study was being conducted. No adverse reactions were reported.
CMS has pushed for the development of institutional standing order sets as a tool to improve compliance with vaccination rate targets. Where the technology is available, computer systems that can screen eligible patients and generate automatic orders are an effective tool in implementing many quality-improvement initiatives, and hospitalists are in a crucial position to take an active role in their development and implementation.
2. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for a trial fibrillation. Ann Intern Med. 2004; 141: 745-52.
Warfarin has been shown to reduce risk of stroke in patients with chronic and paroxysmal atrial fibrillation. Intracranial hemorrhage remains one of the most feared complications of warfarin, especially among older patients, prompting suggestions to consider lower intensity anticoagulation among patients older than 75 years who have atrial fibrillation.
This study evaluated the relationship between the intensity of anticoagulation, risk of intracranial hemorrhage, and age of patients with atrial fibrillation.
This was a retrospective case control study conducted at a tertiary care medical center. One-hundred and seventy patients on warfarin and admitted with intracranial hemorrhage from 1993 to 2002 were matched with 1020 patients who were on warfarin but without intracranial bleed. After controlling for comorbid conditions and aspirin use, authors conducted multivariable logistic regression analysis to determine the odds of intracranial hemorrhage with regard to age and INR. The risk of intracranial hemorrhage increased at 85 years of age and at INR values of 3.5 or greater. The risk of intracranial hemorrhage at INR less than 2.0 did not differ statistically from the risk at INR of 2.0–3.0.
This study shows the risk of intracranial hemorrhage is not decreased by choosing lower intensity anticoagulation, and target INR should still be kept at 2.5 among elderly patients. However, patients older than 85 years should be counseled about their higher risk of intracranial hemorrhage.
3. Heeschen C, Hamm CW, Mitrovic V, et al. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110: 3206-12.
Recent data demonstrate the prognostic value of assessment of neurohormonal activation in patients with acute coronary syndromes (ACS). B-type natriuretic peptide levels (BNP) and levels of the N-terminal fragment of the BNP prohormone (NT-proBNP) predict adverse long-term outcomes in patients with ACS. Investigators reviewed plasma samples of Troponin T (TnT) and NT-proBNP obtained from patients with ACS enrolled in the Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) trial, which randomized patients to tirofiban or heparin for 48 hours and assessed mortality and myocardial infarction at 30 day follow-up. TnT and NT-proBNP determinations were available at baseline for 1791 patients, and at 48 and 72 hours from 1401 patients. Baseline NT-proBNP levels >250 ng/L were associated with significantly higher rates of death and myocardial infarction at 7 and 30 day follow-up. After adjustment for TnT and C-reactive protein levels, elevated NTproBNP levels maintained its predictive value (OR 2.7; p<.001). In patients with normal TnT levels, NT-proBNP levels identified a subgroup of patients at increased risk (OR 3.0; p=.004). However, in patients with high TnT levels (>0.1 mcg/L), NT-proBNP lost its predictive value (p=.58). More importantly, patients with normal levels of both TnT and NT-proBNP were at very low risk (0.6% event rate at 30 day follow-up).
Serial determinations of NT-proBNP levels at 48 and 72 hours were reviewed in patients without major adverse cardiac events (death or myocardial infarction); these patients were subdivided into groups with and without refractory ischemia. Patients without refractory ischemia showed a significant decline in NT-proBNP levels, whereas patients with refractory ischemia had no significant change. Persistently elevated NT-proBNP levels at 72 hours were associated with a 17.2% risk of death or MI at 30 days, compared with 0.6% risk if NT-proBNP returned to normal at 72 hours (p<.001). Neither TnT nor C-reactive protein demonstrated similar predictive value.
The study is limited by its retrospective nature, by potential selection bias by including only patients with direct evidence of coronary artery disease, and by limitations of the generalizability of its findings (e.g., to emergency department patients with chest pain).
As BNP and NT-proBNP are counter-regulatory hormones that play an active role in the response to ischemic injury, the authors suggest that NT-proBNP is a promising tool for dynamic risk assessment in patients with ACS. The authors also do not differentiate between BNP and NT-proBNP with regard to use in risk stratification, which might lead one to believe that these tests share similar predictive value. (Of note, the study was entirely funded by a company that produces an assay for NT-proBNP). Prospective trials to validate this tool are warranted
4. Heuschmann PU, Kolominsky-Rabas PL, Roether J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292:1831-38.
The objective of this study was to identify factors associated with in-hospital mortality in ischemic stroke patients treated with recombinant tissue plasminogen activator (tPA). It was a prospective observational cohort study of 1658 patients conducted at 225 community and academic hospitals throughout Germany with main outcome of in-hospital mortality.
In this study 10% of patients who were treated with tPA died during their hospital stay, with 2/3 of deaths occurring in the first 7 days. Relative probability of in-hospital mortality increased with increasing patient age, with an odds ratio (OR) of 1.6 for each 10-year increment in age. Age was an independent predictor of in hospital mortality irrespective of tPA administration, with patients older than 75 years age having 4 fold higher mortality than the youngest cohort of less than 55 years age.
Other factors predicting in hospital mortality were altered level of consciousness and relative lack of experience with tPA treatment in the center. Altered level of consciousness was a predictor of stroke severity and an independent predictor of in-hospital mortality (OR 3.4). The increase in mortality in centers with limited experience with tPA administration (OR 0.97) reflected learning curve issues with these patients. The study was not designed to separate out the confounders of operator experience curve from institutional experience curves, or to derive the exact relationship between experience and outcomes.
5. McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after non-thoracic surgery. Am J Respir Crit Care Med. 2004; published ahead of print on November 24, 2004 as doi:10.1164/rccm.200408-1069OC. Accessed January 27, 2005.
Postoperative pulmonary complications after nonthoracic surgery are a cause of significant morbidity and increased length of hospital stay. Previous studies of preoperative pulmonary assessment were limited by non-representative patient samples, conflicting results, and lack of explicit definitions of these complications. The authors conducted a prospective cohort study of 1055 patients seen in a Pre-Admission Clinic of a tertiary care university hospital. Mean age was 55 years, 50% male, and the cohort consisted of patients scheduled for intermediate risk elective surgery (upper abdominal, lower abdominal, orthopedic). They evaluated physical exam maneuvers (cough test, wheeze test, maximal laryngeal height, and forced expiratory time, all of which are described in an online data supplement) and preoperative spirometry values, and collected information on clinically significant postoperative pulmonary complications, including pneumonia, respiratory failure requiring mechanical ventilation, atelectasis requiring bronchoscopy, or pneumothorax or pleural effusion requiring percutaneous intervention. Twenty-eight patients (2.7%) suffered a pulmonary complication within 7 days of surgery, one of whom died. Length of stay was significantly prolonged in this group (mean 27.9 days vs 4.5 days, p=.006). Multivariate regression analysis revealed four variables that were independently associated with increased risk for postoperative pulmonary complications: age > 65 years, positive cough test (repeated coughing after asking the patient to inhale deeply and cough once), perioperative nasogastric tube, and anesthesia duration 2.5 hours or greater. Number of pack years smoked, FEV1, FEV1/FVC ratio, and upper abdominal surgery were associated with postoperative pulmonary complications but were not found to be independently associated by multivariate analysis.
While it is not surprising that the above risk factors are predictive of postoperative complications, this is the first study to incorporate specific exam maneuvers and spirometry into a risk prediction analysis. Limitations of this model are lack of independent validation and lack of generalizability to other populations, e.g., inpatients awaiting urgent surgery. Of note, the study further provides further support for not routinely obtaining pulmonary function testing for risk stratification prior to noncardiac surgery.
6. Mortenson, EM, Restrepo M, Anzeuto A, Pugh J. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med. 2004;117:726-31.
The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) have published guidelines for the management of community acquired pneumonia that include recommendations for antibiotic selection. This retrospective cohort study attempted to measure the association between adherence to such guidelines and 30-day mortality in patients admitted with pneumonia to two Texas hospitals.
The characteristics of the patients studied reflect a reasonable cross-section of typical pneumonia patients, with the exception that the patients were mostly (85%) men. Of the study population, 78% were admitted through the ER, 20% admitted to the ICU, and 9% were nursing home residents. Antibiotics were considered “concordant” if they were consistent with either the most recent IDSA or ATS recommendations. The “nonconcordant” group was slightly older (66 vs. 61), generally sicker (higher rate of comorbid COPD and CVD), had more cerebrovascular disease, was more likely to present with altered mental status, and less frequently received antibiotics within 8 hours of presentation. The study did not comment on patients’ vaccination status. Thirty-day mortality was 6.2% in the guideline-concordant group, versus 21.7% in the other group (p < .001). The most common “non-concordant” regimen described was use of a beta-lactam alone, although specific antibiotic regimens were not evaluated.
While the results of this study are not surprising, they provide us with both the validation to continue practice according to existing recommendations, particularly the avoidance of monotherapy with beta-lactam antibiotics. The study also provides us with the imperative to take the lead in developing evidence-based pneumonia pathways at our own hospitals.
7. Pham MX, Whooley MA, Evans GT Jr, et al. Prognostic value of low-level cardiac troponin-I elevations in patients without definite acute coronary syndromes. Am Heart J. 2004;148: 776-82.
With the availability of rapid and highly sensitive and specific troponin testing, many patients admitted to the hospital with non-cardiac diagnoses have been recognized as having abnormal serum troponin-I or troponin-T levels, often just slightly above the reference cutoff for “normal.” While the clinical assumption is often that the elevated enzyme level does not reflect an acute coronary syndrome per se, its significance regarding the patient’s underlying cardiac health is often unclear.
Pham et al. retrospectively reviewed the 1-year mortality of 366 patients who were admitted to the San Francisco VA without evidence of acute MI or ACS either clinically or by EKG, but who had low-level troponin-I elevations (up to 3.0 ng/mL—a level that the authors state was reached by institutional consensus, and which was measured by a “first-generation” assay). These patients were admitted for a broad spectrum of diagnoses ranging from CHF to COPD to sepsis. Ninety-six percent of the patients were men; their average age was 69.
Follow-up was accomplished after a mean of 288 days and included 97% of patients. The primary endpoint was MI or death due to cardiac disease at one year; secondary endpoints were revascularization or admission for unstable angina. The primary endpoint was reached by 11% of patients with cTn-I between 1.0 and 3.0 ng/mL, and 4% of the patients with cTn-I up to 1.0 ng/mL (adjusted HR 3.4, 95% CI, 1.3 to 9.4), and the higher the cTn-I, the higher the risk. However, the authors did not test for the level of risk by specific diagnosis, so they caution against overgeneralizing their findings.
The findings of this study add to the evidence that any evidence of myocardial injury implies an increased risk of underlying heart disease and accompanying long—term cardiac complications– even if such injury occurs in the absence of ACS or known heart disease. Hospitalists often see such injury in the setting of acute infection and pulmonary disease and may be the first to recognize the possibility of CHD in a given patient. To date, guidelines addressing optimal prospective risk stratification have not been developed. Until they are, hospitalists should be aware of the ramifications of “troponin leak” and be prepared to initiate necessary inpatient monitoring and treatments, and to coordinate appropriate follow-up for these patients.
8. Saposnik G, Young B, Silver B, et al. Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA. 2004; 292: 1839-44.
Recombinant tissue plasminogen activator (tPA) has been shown to be one of the most efficacious therapies for acute stroke treatment. This was a systematic evaluation of predictors for outcomes at 24 hours after tPA therapy and of the prognostic significance of lack of improvement at 24 hours for long-term outcomes at 3 months.
The trial was a prospective cohort study of 216 consecutive patients admitted with acute ischemic stroke to a university hospital. The decision to treat with tPA was based on the NINDS protocol with one difference: patients with involvement of more than one third of the middle cerebral artery on the baseline CT scan were excluded. A control CT scan was performed at 24 hours to determine the presence of new infarction, cortical involvement, and extension of the ischemic lesion.
Lack of improvement was defined as a difference between the NIHSS score at baseline and at 24 hours of 3 points or less. Poor outcome at 3 months was defined by a modified Rankin Scale score of 3 to 5 or death.
After adjusting for age, gender, and stroke severity, hyperglycemia at admission (glucose > 144 mg/dL), cortical involvement, and time to treatment were independent predictors of lack of improvement at 24 hours. After adjusting for age, gender, and stroke severity, lack of improvement at 24 hours was an independent predictor of poor outcome and death at 3 months. Patients with lack of improvement at 24 hours also had longer lengths of hospitalization.
9. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-57.
Neurohormonal changes, endothelial dysfunction, impaired nitric oxide availability, and oxidant stress all contribute to the structural remodeling of the left ventricle in congestive heart failure. The combination of isosorbide dinitrate, an organic nitrate that stimulates nitric oxide signaling, and the antioxidant and vasodilator hydralazine improves survival in heart failure. Based on more recent data that black patients have a clinically significant response to this combination therapy, the authors of the African-American Heart Failure Trial (A-HeFT) evaluated 1050 black patients with congestive heart failure in a randomized, double-blind, placebo controlled trial. Patients were randomized to fixed doses of isosorbide dinitrate and hydralazine plus background therapy (i.e., digoxin, ACE inhibitors, beta-blockers, diuretics, angiotensin receptor blockers) or to placebo plus background therapy. After 18 months, the trial was stopped due to a significantly higher mortality rate in the placebo group (10.2% in the placebo group vs. 6.2% with combination
therapy, p=.02); survival differences emerged at 180 days and increased progressively thereafter. The combination therapy group reported more headache and dizziness but suffered fewer exacerbations of congestive heart failure and reported improvement in subjective assessments of quality of life as measured by questionnaires. Accompanying editorials discuss the role of nitric oxide and prevention of oxidative stress in the treatment of heart failure, as well as the controversial issues surrounding race-based therapeutics.
1. Dexter PR, Perkins SM, Mahany KS, Jones K, McDonald CJ. Inpatient computer-based standing orders vs. physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA. 2004; 292: 2366-71.
Past studies have suggested that most patients admitted with severe pneumococcal infections have been hospitalized in the preceding 5 years, and simply being hospitalized is a potential risk factor for later pneumococcal infection. Likewise, hospitalization provides an opportunity to vaccinate high-risk patients against influenza, and raising pneumococcal and influenza immunization rates is a CMS quality improvement priority. Prior investigations have supported the use of labor-intensive manual standing orders as well as computerized reminders, but this prospective trial was conducted in 1998 and 1999 to assess the effectiveness of a computer-based system to screen for eligible patients and then generate orders to perform pneumonia and influenza vaccinations on inpatients at the time of discharge.
Over 13 months, a total of 3777 inpatients were entered into the study. The hospital computer identified patients eligible for vaccination based on common criteria and randomized them to one of two groups of physician teams. For one group of teams, the computer order-entry system would automatically generate vaccination orders at the time of discharge for vaccine-eligible patients; for the other group of teams, only computer reminders were provided to physicians. The outcome measure was administration of vaccine; long-term outcomes such as incidence of subsequent disease or mortality were not measured.
During the study period, 50% of all hospitalized patients were identified as eligible for influenza vaccination; 22% were eligible for pneumococcal vaccination. In each case, the “standing order” group received vaccine more often (influenza: 42% vs. 30%, p<.001; pneumococcal vaccine: 51% vs. 31%). The numbers were subsequently adjusted to allow for patients who had previously received vaccine, but the impressive differences persisted. Nurses reported reasons for non-administration in 98% of the eligible patients who were not vaccinated; the most common reason was patient refusal. It is not clear if the physicians knew that a study was being conducted. No adverse reactions were reported.
CMS has pushed for the development of institutional standing order sets as a tool to improve compliance with vaccination rate targets. Where the technology is available, computer systems that can screen eligible patients and generate automatic orders are an effective tool in implementing many quality-improvement initiatives, and hospitalists are in a crucial position to take an active role in their development and implementation.
2. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for a trial fibrillation. Ann Intern Med. 2004; 141: 745-52.
Warfarin has been shown to reduce risk of stroke in patients with chronic and paroxysmal atrial fibrillation. Intracranial hemorrhage remains one of the most feared complications of warfarin, especially among older patients, prompting suggestions to consider lower intensity anticoagulation among patients older than 75 years who have atrial fibrillation.
This study evaluated the relationship between the intensity of anticoagulation, risk of intracranial hemorrhage, and age of patients with atrial fibrillation.
This was a retrospective case control study conducted at a tertiary care medical center. One-hundred and seventy patients on warfarin and admitted with intracranial hemorrhage from 1993 to 2002 were matched with 1020 patients who were on warfarin but without intracranial bleed. After controlling for comorbid conditions and aspirin use, authors conducted multivariable logistic regression analysis to determine the odds of intracranial hemorrhage with regard to age and INR. The risk of intracranial hemorrhage increased at 85 years of age and at INR values of 3.5 or greater. The risk of intracranial hemorrhage at INR less than 2.0 did not differ statistically from the risk at INR of 2.0–3.0.
This study shows the risk of intracranial hemorrhage is not decreased by choosing lower intensity anticoagulation, and target INR should still be kept at 2.5 among elderly patients. However, patients older than 85 years should be counseled about their higher risk of intracranial hemorrhage.
3. Heeschen C, Hamm CW, Mitrovic V, et al. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110: 3206-12.
Recent data demonstrate the prognostic value of assessment of neurohormonal activation in patients with acute coronary syndromes (ACS). B-type natriuretic peptide levels (BNP) and levels of the N-terminal fragment of the BNP prohormone (NT-proBNP) predict adverse long-term outcomes in patients with ACS. Investigators reviewed plasma samples of Troponin T (TnT) and NT-proBNP obtained from patients with ACS enrolled in the Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) trial, which randomized patients to tirofiban or heparin for 48 hours and assessed mortality and myocardial infarction at 30 day follow-up. TnT and NT-proBNP determinations were available at baseline for 1791 patients, and at 48 and 72 hours from 1401 patients. Baseline NT-proBNP levels >250 ng/L were associated with significantly higher rates of death and myocardial infarction at 7 and 30 day follow-up. After adjustment for TnT and C-reactive protein levels, elevated NTproBNP levels maintained its predictive value (OR 2.7; p<.001). In patients with normal TnT levels, NT-proBNP levels identified a subgroup of patients at increased risk (OR 3.0; p=.004). However, in patients with high TnT levels (>0.1 mcg/L), NT-proBNP lost its predictive value (p=.58). More importantly, patients with normal levels of both TnT and NT-proBNP were at very low risk (0.6% event rate at 30 day follow-up).
Serial determinations of NT-proBNP levels at 48 and 72 hours were reviewed in patients without major adverse cardiac events (death or myocardial infarction); these patients were subdivided into groups with and without refractory ischemia. Patients without refractory ischemia showed a significant decline in NT-proBNP levels, whereas patients with refractory ischemia had no significant change. Persistently elevated NT-proBNP levels at 72 hours were associated with a 17.2% risk of death or MI at 30 days, compared with 0.6% risk if NT-proBNP returned to normal at 72 hours (p<.001). Neither TnT nor C-reactive protein demonstrated similar predictive value.
The study is limited by its retrospective nature, by potential selection bias by including only patients with direct evidence of coronary artery disease, and by limitations of the generalizability of its findings (e.g., to emergency department patients with chest pain).
As BNP and NT-proBNP are counter-regulatory hormones that play an active role in the response to ischemic injury, the authors suggest that NT-proBNP is a promising tool for dynamic risk assessment in patients with ACS. The authors also do not differentiate between BNP and NT-proBNP with regard to use in risk stratification, which might lead one to believe that these tests share similar predictive value. (Of note, the study was entirely funded by a company that produces an assay for NT-proBNP). Prospective trials to validate this tool are warranted
4. Heuschmann PU, Kolominsky-Rabas PL, Roether J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292:1831-38.
The objective of this study was to identify factors associated with in-hospital mortality in ischemic stroke patients treated with recombinant tissue plasminogen activator (tPA). It was a prospective observational cohort study of 1658 patients conducted at 225 community and academic hospitals throughout Germany with main outcome of in-hospital mortality.
In this study 10% of patients who were treated with tPA died during their hospital stay, with 2/3 of deaths occurring in the first 7 days. Relative probability of in-hospital mortality increased with increasing patient age, with an odds ratio (OR) of 1.6 for each 10-year increment in age. Age was an independent predictor of in hospital mortality irrespective of tPA administration, with patients older than 75 years age having 4 fold higher mortality than the youngest cohort of less than 55 years age.
Other factors predicting in hospital mortality were altered level of consciousness and relative lack of experience with tPA treatment in the center. Altered level of consciousness was a predictor of stroke severity and an independent predictor of in-hospital mortality (OR 3.4). The increase in mortality in centers with limited experience with tPA administration (OR 0.97) reflected learning curve issues with these patients. The study was not designed to separate out the confounders of operator experience curve from institutional experience curves, or to derive the exact relationship between experience and outcomes.
5. McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after non-thoracic surgery. Am J Respir Crit Care Med. 2004; published ahead of print on November 24, 2004 as doi:10.1164/rccm.200408-1069OC. Accessed January 27, 2005.
Postoperative pulmonary complications after nonthoracic surgery are a cause of significant morbidity and increased length of hospital stay. Previous studies of preoperative pulmonary assessment were limited by non-representative patient samples, conflicting results, and lack of explicit definitions of these complications. The authors conducted a prospective cohort study of 1055 patients seen in a Pre-Admission Clinic of a tertiary care university hospital. Mean age was 55 years, 50% male, and the cohort consisted of patients scheduled for intermediate risk elective surgery (upper abdominal, lower abdominal, orthopedic). They evaluated physical exam maneuvers (cough test, wheeze test, maximal laryngeal height, and forced expiratory time, all of which are described in an online data supplement) and preoperative spirometry values, and collected information on clinically significant postoperative pulmonary complications, including pneumonia, respiratory failure requiring mechanical ventilation, atelectasis requiring bronchoscopy, or pneumothorax or pleural effusion requiring percutaneous intervention. Twenty-eight patients (2.7%) suffered a pulmonary complication within 7 days of surgery, one of whom died. Length of stay was significantly prolonged in this group (mean 27.9 days vs 4.5 days, p=.006). Multivariate regression analysis revealed four variables that were independently associated with increased risk for postoperative pulmonary complications: age > 65 years, positive cough test (repeated coughing after asking the patient to inhale deeply and cough once), perioperative nasogastric tube, and anesthesia duration 2.5 hours or greater. Number of pack years smoked, FEV1, FEV1/FVC ratio, and upper abdominal surgery were associated with postoperative pulmonary complications but were not found to be independently associated by multivariate analysis.
While it is not surprising that the above risk factors are predictive of postoperative complications, this is the first study to incorporate specific exam maneuvers and spirometry into a risk prediction analysis. Limitations of this model are lack of independent validation and lack of generalizability to other populations, e.g., inpatients awaiting urgent surgery. Of note, the study further provides further support for not routinely obtaining pulmonary function testing for risk stratification prior to noncardiac surgery.
6. Mortenson, EM, Restrepo M, Anzeuto A, Pugh J. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med. 2004;117:726-31.
The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) have published guidelines for the management of community acquired pneumonia that include recommendations for antibiotic selection. This retrospective cohort study attempted to measure the association between adherence to such guidelines and 30-day mortality in patients admitted with pneumonia to two Texas hospitals.
The characteristics of the patients studied reflect a reasonable cross-section of typical pneumonia patients, with the exception that the patients were mostly (85%) men. Of the study population, 78% were admitted through the ER, 20% admitted to the ICU, and 9% were nursing home residents. Antibiotics were considered “concordant” if they were consistent with either the most recent IDSA or ATS recommendations. The “nonconcordant” group was slightly older (66 vs. 61), generally sicker (higher rate of comorbid COPD and CVD), had more cerebrovascular disease, was more likely to present with altered mental status, and less frequently received antibiotics within 8 hours of presentation. The study did not comment on patients’ vaccination status. Thirty-day mortality was 6.2% in the guideline-concordant group, versus 21.7% in the other group (p < .001). The most common “non-concordant” regimen described was use of a beta-lactam alone, although specific antibiotic regimens were not evaluated.
While the results of this study are not surprising, they provide us with both the validation to continue practice according to existing recommendations, particularly the avoidance of monotherapy with beta-lactam antibiotics. The study also provides us with the imperative to take the lead in developing evidence-based pneumonia pathways at our own hospitals.
7. Pham MX, Whooley MA, Evans GT Jr, et al. Prognostic value of low-level cardiac troponin-I elevations in patients without definite acute coronary syndromes. Am Heart J. 2004;148: 776-82.
With the availability of rapid and highly sensitive and specific troponin testing, many patients admitted to the hospital with non-cardiac diagnoses have been recognized as having abnormal serum troponin-I or troponin-T levels, often just slightly above the reference cutoff for “normal.” While the clinical assumption is often that the elevated enzyme level does not reflect an acute coronary syndrome per se, its significance regarding the patient’s underlying cardiac health is often unclear.
Pham et al. retrospectively reviewed the 1-year mortality of 366 patients who were admitted to the San Francisco VA without evidence of acute MI or ACS either clinically or by EKG, but who had low-level troponin-I elevations (up to 3.0 ng/mL—a level that the authors state was reached by institutional consensus, and which was measured by a “first-generation” assay). These patients were admitted for a broad spectrum of diagnoses ranging from CHF to COPD to sepsis. Ninety-six percent of the patients were men; their average age was 69.
Follow-up was accomplished after a mean of 288 days and included 97% of patients. The primary endpoint was MI or death due to cardiac disease at one year; secondary endpoints were revascularization or admission for unstable angina. The primary endpoint was reached by 11% of patients with cTn-I between 1.0 and 3.0 ng/mL, and 4% of the patients with cTn-I up to 1.0 ng/mL (adjusted HR 3.4, 95% CI, 1.3 to 9.4), and the higher the cTn-I, the higher the risk. However, the authors did not test for the level of risk by specific diagnosis, so they caution against overgeneralizing their findings.
The findings of this study add to the evidence that any evidence of myocardial injury implies an increased risk of underlying heart disease and accompanying long—term cardiac complications– even if such injury occurs in the absence of ACS or known heart disease. Hospitalists often see such injury in the setting of acute infection and pulmonary disease and may be the first to recognize the possibility of CHD in a given patient. To date, guidelines addressing optimal prospective risk stratification have not been developed. Until they are, hospitalists should be aware of the ramifications of “troponin leak” and be prepared to initiate necessary inpatient monitoring and treatments, and to coordinate appropriate follow-up for these patients.
8. Saposnik G, Young B, Silver B, et al. Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA. 2004; 292: 1839-44.
Recombinant tissue plasminogen activator (tPA) has been shown to be one of the most efficacious therapies for acute stroke treatment. This was a systematic evaluation of predictors for outcomes at 24 hours after tPA therapy and of the prognostic significance of lack of improvement at 24 hours for long-term outcomes at 3 months.
The trial was a prospective cohort study of 216 consecutive patients admitted with acute ischemic stroke to a university hospital. The decision to treat with tPA was based on the NINDS protocol with one difference: patients with involvement of more than one third of the middle cerebral artery on the baseline CT scan were excluded. A control CT scan was performed at 24 hours to determine the presence of new infarction, cortical involvement, and extension of the ischemic lesion.
Lack of improvement was defined as a difference between the NIHSS score at baseline and at 24 hours of 3 points or less. Poor outcome at 3 months was defined by a modified Rankin Scale score of 3 to 5 or death.
After adjusting for age, gender, and stroke severity, hyperglycemia at admission (glucose > 144 mg/dL), cortical involvement, and time to treatment were independent predictors of lack of improvement at 24 hours. After adjusting for age, gender, and stroke severity, lack of improvement at 24 hours was an independent predictor of poor outcome and death at 3 months. Patients with lack of improvement at 24 hours also had longer lengths of hospitalization.
9. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-57.
Neurohormonal changes, endothelial dysfunction, impaired nitric oxide availability, and oxidant stress all contribute to the structural remodeling of the left ventricle in congestive heart failure. The combination of isosorbide dinitrate, an organic nitrate that stimulates nitric oxide signaling, and the antioxidant and vasodilator hydralazine improves survival in heart failure. Based on more recent data that black patients have a clinically significant response to this combination therapy, the authors of the African-American Heart Failure Trial (A-HeFT) evaluated 1050 black patients with congestive heart failure in a randomized, double-blind, placebo controlled trial. Patients were randomized to fixed doses of isosorbide dinitrate and hydralazine plus background therapy (i.e., digoxin, ACE inhibitors, beta-blockers, diuretics, angiotensin receptor blockers) or to placebo plus background therapy. After 18 months, the trial was stopped due to a significantly higher mortality rate in the placebo group (10.2% in the placebo group vs. 6.2% with combination
therapy, p=.02); survival differences emerged at 180 days and increased progressively thereafter. The combination therapy group reported more headache and dizziness but suffered fewer exacerbations of congestive heart failure and reported improvement in subjective assessments of quality of life as measured by questionnaires. Accompanying editorials discuss the role of nitric oxide and prevention of oxidative stress in the treatment of heart failure, as well as the controversial issues surrounding race-based therapeutics.
1. Dexter PR, Perkins SM, Mahany KS, Jones K, McDonald CJ. Inpatient computer-based standing orders vs. physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA. 2004; 292: 2366-71.
Past studies have suggested that most patients admitted with severe pneumococcal infections have been hospitalized in the preceding 5 years, and simply being hospitalized is a potential risk factor for later pneumococcal infection. Likewise, hospitalization provides an opportunity to vaccinate high-risk patients against influenza, and raising pneumococcal and influenza immunization rates is a CMS quality improvement priority. Prior investigations have supported the use of labor-intensive manual standing orders as well as computerized reminders, but this prospective trial was conducted in 1998 and 1999 to assess the effectiveness of a computer-based system to screen for eligible patients and then generate orders to perform pneumonia and influenza vaccinations on inpatients at the time of discharge.
Over 13 months, a total of 3777 inpatients were entered into the study. The hospital computer identified patients eligible for vaccination based on common criteria and randomized them to one of two groups of physician teams. For one group of teams, the computer order-entry system would automatically generate vaccination orders at the time of discharge for vaccine-eligible patients; for the other group of teams, only computer reminders were provided to physicians. The outcome measure was administration of vaccine; long-term outcomes such as incidence of subsequent disease or mortality were not measured.
During the study period, 50% of all hospitalized patients were identified as eligible for influenza vaccination; 22% were eligible for pneumococcal vaccination. In each case, the “standing order” group received vaccine more often (influenza: 42% vs. 30%, p<.001; pneumococcal vaccine: 51% vs. 31%). The numbers were subsequently adjusted to allow for patients who had previously received vaccine, but the impressive differences persisted. Nurses reported reasons for non-administration in 98% of the eligible patients who were not vaccinated; the most common reason was patient refusal. It is not clear if the physicians knew that a study was being conducted. No adverse reactions were reported.
CMS has pushed for the development of institutional standing order sets as a tool to improve compliance with vaccination rate targets. Where the technology is available, computer systems that can screen eligible patients and generate automatic orders are an effective tool in implementing many quality-improvement initiatives, and hospitalists are in a crucial position to take an active role in their development and implementation.
2. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for a trial fibrillation. Ann Intern Med. 2004; 141: 745-52.
Warfarin has been shown to reduce risk of stroke in patients with chronic and paroxysmal atrial fibrillation. Intracranial hemorrhage remains one of the most feared complications of warfarin, especially among older patients, prompting suggestions to consider lower intensity anticoagulation among patients older than 75 years who have atrial fibrillation.
This study evaluated the relationship between the intensity of anticoagulation, risk of intracranial hemorrhage, and age of patients with atrial fibrillation.
This was a retrospective case control study conducted at a tertiary care medical center. One-hundred and seventy patients on warfarin and admitted with intracranial hemorrhage from 1993 to 2002 were matched with 1020 patients who were on warfarin but without intracranial bleed. After controlling for comorbid conditions and aspirin use, authors conducted multivariable logistic regression analysis to determine the odds of intracranial hemorrhage with regard to age and INR. The risk of intracranial hemorrhage increased at 85 years of age and at INR values of 3.5 or greater. The risk of intracranial hemorrhage at INR less than 2.0 did not differ statistically from the risk at INR of 2.0–3.0.
This study shows the risk of intracranial hemorrhage is not decreased by choosing lower intensity anticoagulation, and target INR should still be kept at 2.5 among elderly patients. However, patients older than 85 years should be counseled about their higher risk of intracranial hemorrhage.
3. Heeschen C, Hamm CW, Mitrovic V, et al. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110: 3206-12.
Recent data demonstrate the prognostic value of assessment of neurohormonal activation in patients with acute coronary syndromes (ACS). B-type natriuretic peptide levels (BNP) and levels of the N-terminal fragment of the BNP prohormone (NT-proBNP) predict adverse long-term outcomes in patients with ACS. Investigators reviewed plasma samples of Troponin T (TnT) and NT-proBNP obtained from patients with ACS enrolled in the Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) trial, which randomized patients to tirofiban or heparin for 48 hours and assessed mortality and myocardial infarction at 30 day follow-up. TnT and NT-proBNP determinations were available at baseline for 1791 patients, and at 48 and 72 hours from 1401 patients. Baseline NT-proBNP levels >250 ng/L were associated with significantly higher rates of death and myocardial infarction at 7 and 30 day follow-up. After adjustment for TnT and C-reactive protein levels, elevated NTproBNP levels maintained its predictive value (OR 2.7; p<.001). In patients with normal TnT levels, NT-proBNP levels identified a subgroup of patients at increased risk (OR 3.0; p=.004). However, in patients with high TnT levels (>0.1 mcg/L), NT-proBNP lost its predictive value (p=.58). More importantly, patients with normal levels of both TnT and NT-proBNP were at very low risk (0.6% event rate at 30 day follow-up).
Serial determinations of NT-proBNP levels at 48 and 72 hours were reviewed in patients without major adverse cardiac events (death or myocardial infarction); these patients were subdivided into groups with and without refractory ischemia. Patients without refractory ischemia showed a significant decline in NT-proBNP levels, whereas patients with refractory ischemia had no significant change. Persistently elevated NT-proBNP levels at 72 hours were associated with a 17.2% risk of death or MI at 30 days, compared with 0.6% risk if NT-proBNP returned to normal at 72 hours (p<.001). Neither TnT nor C-reactive protein demonstrated similar predictive value.
The study is limited by its retrospective nature, by potential selection bias by including only patients with direct evidence of coronary artery disease, and by limitations of the generalizability of its findings (e.g., to emergency department patients with chest pain).
As BNP and NT-proBNP are counter-regulatory hormones that play an active role in the response to ischemic injury, the authors suggest that NT-proBNP is a promising tool for dynamic risk assessment in patients with ACS. The authors also do not differentiate between BNP and NT-proBNP with regard to use in risk stratification, which might lead one to believe that these tests share similar predictive value. (Of note, the study was entirely funded by a company that produces an assay for NT-proBNP). Prospective trials to validate this tool are warranted
4. Heuschmann PU, Kolominsky-Rabas PL, Roether J, et al. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA. 2004;292:1831-38.
The objective of this study was to identify factors associated with in-hospital mortality in ischemic stroke patients treated with recombinant tissue plasminogen activator (tPA). It was a prospective observational cohort study of 1658 patients conducted at 225 community and academic hospitals throughout Germany with main outcome of in-hospital mortality.
In this study 10% of patients who were treated with tPA died during their hospital stay, with 2/3 of deaths occurring in the first 7 days. Relative probability of in-hospital mortality increased with increasing patient age, with an odds ratio (OR) of 1.6 for each 10-year increment in age. Age was an independent predictor of in hospital mortality irrespective of tPA administration, with patients older than 75 years age having 4 fold higher mortality than the youngest cohort of less than 55 years age.
Other factors predicting in hospital mortality were altered level of consciousness and relative lack of experience with tPA treatment in the center. Altered level of consciousness was a predictor of stroke severity and an independent predictor of in-hospital mortality (OR 3.4). The increase in mortality in centers with limited experience with tPA administration (OR 0.97) reflected learning curve issues with these patients. The study was not designed to separate out the confounders of operator experience curve from institutional experience curves, or to derive the exact relationship between experience and outcomes.
5. McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after non-thoracic surgery. Am J Respir Crit Care Med. 2004; published ahead of print on November 24, 2004 as doi:10.1164/rccm.200408-1069OC. Accessed January 27, 2005.
Postoperative pulmonary complications after nonthoracic surgery are a cause of significant morbidity and increased length of hospital stay. Previous studies of preoperative pulmonary assessment were limited by non-representative patient samples, conflicting results, and lack of explicit definitions of these complications. The authors conducted a prospective cohort study of 1055 patients seen in a Pre-Admission Clinic of a tertiary care university hospital. Mean age was 55 years, 50% male, and the cohort consisted of patients scheduled for intermediate risk elective surgery (upper abdominal, lower abdominal, orthopedic). They evaluated physical exam maneuvers (cough test, wheeze test, maximal laryngeal height, and forced expiratory time, all of which are described in an online data supplement) and preoperative spirometry values, and collected information on clinically significant postoperative pulmonary complications, including pneumonia, respiratory failure requiring mechanical ventilation, atelectasis requiring bronchoscopy, or pneumothorax or pleural effusion requiring percutaneous intervention. Twenty-eight patients (2.7%) suffered a pulmonary complication within 7 days of surgery, one of whom died. Length of stay was significantly prolonged in this group (mean 27.9 days vs 4.5 days, p=.006). Multivariate regression analysis revealed four variables that were independently associated with increased risk for postoperative pulmonary complications: age > 65 years, positive cough test (repeated coughing after asking the patient to inhale deeply and cough once), perioperative nasogastric tube, and anesthesia duration 2.5 hours or greater. Number of pack years smoked, FEV1, FEV1/FVC ratio, and upper abdominal surgery were associated with postoperative pulmonary complications but were not found to be independently associated by multivariate analysis.
While it is not surprising that the above risk factors are predictive of postoperative complications, this is the first study to incorporate specific exam maneuvers and spirometry into a risk prediction analysis. Limitations of this model are lack of independent validation and lack of generalizability to other populations, e.g., inpatients awaiting urgent surgery. Of note, the study further provides further support for not routinely obtaining pulmonary function testing for risk stratification prior to noncardiac surgery.
6. Mortenson, EM, Restrepo M, Anzeuto A, Pugh J. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med. 2004;117:726-31.
The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) have published guidelines for the management of community acquired pneumonia that include recommendations for antibiotic selection. This retrospective cohort study attempted to measure the association between adherence to such guidelines and 30-day mortality in patients admitted with pneumonia to two Texas hospitals.
The characteristics of the patients studied reflect a reasonable cross-section of typical pneumonia patients, with the exception that the patients were mostly (85%) men. Of the study population, 78% were admitted through the ER, 20% admitted to the ICU, and 9% were nursing home residents. Antibiotics were considered “concordant” if they were consistent with either the most recent IDSA or ATS recommendations. The “nonconcordant” group was slightly older (66 vs. 61), generally sicker (higher rate of comorbid COPD and CVD), had more cerebrovascular disease, was more likely to present with altered mental status, and less frequently received antibiotics within 8 hours of presentation. The study did not comment on patients’ vaccination status. Thirty-day mortality was 6.2% in the guideline-concordant group, versus 21.7% in the other group (p < .001). The most common “non-concordant” regimen described was use of a beta-lactam alone, although specific antibiotic regimens were not evaluated.
While the results of this study are not surprising, they provide us with both the validation to continue practice according to existing recommendations, particularly the avoidance of monotherapy with beta-lactam antibiotics. The study also provides us with the imperative to take the lead in developing evidence-based pneumonia pathways at our own hospitals.
7. Pham MX, Whooley MA, Evans GT Jr, et al. Prognostic value of low-level cardiac troponin-I elevations in patients without definite acute coronary syndromes. Am Heart J. 2004;148: 776-82.
With the availability of rapid and highly sensitive and specific troponin testing, many patients admitted to the hospital with non-cardiac diagnoses have been recognized as having abnormal serum troponin-I or troponin-T levels, often just slightly above the reference cutoff for “normal.” While the clinical assumption is often that the elevated enzyme level does not reflect an acute coronary syndrome per se, its significance regarding the patient’s underlying cardiac health is often unclear.
Pham et al. retrospectively reviewed the 1-year mortality of 366 patients who were admitted to the San Francisco VA without evidence of acute MI or ACS either clinically or by EKG, but who had low-level troponin-I elevations (up to 3.0 ng/mL—a level that the authors state was reached by institutional consensus, and which was measured by a “first-generation” assay). These patients were admitted for a broad spectrum of diagnoses ranging from CHF to COPD to sepsis. Ninety-six percent of the patients were men; their average age was 69.
Follow-up was accomplished after a mean of 288 days and included 97% of patients. The primary endpoint was MI or death due to cardiac disease at one year; secondary endpoints were revascularization or admission for unstable angina. The primary endpoint was reached by 11% of patients with cTn-I between 1.0 and 3.0 ng/mL, and 4% of the patients with cTn-I up to 1.0 ng/mL (adjusted HR 3.4, 95% CI, 1.3 to 9.4), and the higher the cTn-I, the higher the risk. However, the authors did not test for the level of risk by specific diagnosis, so they caution against overgeneralizing their findings.
The findings of this study add to the evidence that any evidence of myocardial injury implies an increased risk of underlying heart disease and accompanying long—term cardiac complications– even if such injury occurs in the absence of ACS or known heart disease. Hospitalists often see such injury in the setting of acute infection and pulmonary disease and may be the first to recognize the possibility of CHD in a given patient. To date, guidelines addressing optimal prospective risk stratification have not been developed. Until they are, hospitalists should be aware of the ramifications of “troponin leak” and be prepared to initiate necessary inpatient monitoring and treatments, and to coordinate appropriate follow-up for these patients.
8. Saposnik G, Young B, Silver B, et al. Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA. 2004; 292: 1839-44.
Recombinant tissue plasminogen activator (tPA) has been shown to be one of the most efficacious therapies for acute stroke treatment. This was a systematic evaluation of predictors for outcomes at 24 hours after tPA therapy and of the prognostic significance of lack of improvement at 24 hours for long-term outcomes at 3 months.
The trial was a prospective cohort study of 216 consecutive patients admitted with acute ischemic stroke to a university hospital. The decision to treat with tPA was based on the NINDS protocol with one difference: patients with involvement of more than one third of the middle cerebral artery on the baseline CT scan were excluded. A control CT scan was performed at 24 hours to determine the presence of new infarction, cortical involvement, and extension of the ischemic lesion.
Lack of improvement was defined as a difference between the NIHSS score at baseline and at 24 hours of 3 points or less. Poor outcome at 3 months was defined by a modified Rankin Scale score of 3 to 5 or death.
After adjusting for age, gender, and stroke severity, hyperglycemia at admission (glucose > 144 mg/dL), cortical involvement, and time to treatment were independent predictors of lack of improvement at 24 hours. After adjusting for age, gender, and stroke severity, lack of improvement at 24 hours was an independent predictor of poor outcome and death at 3 months. Patients with lack of improvement at 24 hours also had longer lengths of hospitalization.
9. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-57.
Neurohormonal changes, endothelial dysfunction, impaired nitric oxide availability, and oxidant stress all contribute to the structural remodeling of the left ventricle in congestive heart failure. The combination of isosorbide dinitrate, an organic nitrate that stimulates nitric oxide signaling, and the antioxidant and vasodilator hydralazine improves survival in heart failure. Based on more recent data that black patients have a clinically significant response to this combination therapy, the authors of the African-American Heart Failure Trial (A-HeFT) evaluated 1050 black patients with congestive heart failure in a randomized, double-blind, placebo controlled trial. Patients were randomized to fixed doses of isosorbide dinitrate and hydralazine plus background therapy (i.e., digoxin, ACE inhibitors, beta-blockers, diuretics, angiotensin receptor blockers) or to placebo plus background therapy. After 18 months, the trial was stopped due to a significantly higher mortality rate in the placebo group (10.2% in the placebo group vs. 6.2% with combination
therapy, p=.02); survival differences emerged at 180 days and increased progressively thereafter. The combination therapy group reported more headache and dizziness but suffered fewer exacerbations of congestive heart failure and reported improvement in subjective assessments of quality of life as measured by questionnaires. Accompanying editorials discuss the role of nitric oxide and prevention of oxidative stress in the treatment of heart failure, as well as the controversial issues surrounding race-based therapeutics.
In the Literature
CARP Trial Suggests No Benefit to Revascularization Before Vascular Surgery
McFalls, EO, Ward HB, Mortiz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
Recent studies have presented evidence that treatment with beta-blockers for patients with CAD could reduce the risk of perioperative cardiac complications. Beta-blockers have since become a critical part of the management plan for the perioperative patient. Evidence-based practice guidelines for cardiac risk assessment have been published by both the American College of Physicians and the American College of Cardiology/American Heart Association. However, practice patterns continue to vary between physicians and cardiologists, particularly for patients clinically stratified into the intermediate-risk category. Some physicians feel comfortable with a conservative approach of medical optimization even in the setting of established CAD, while others favor more aggressive treatment, even though the prospective data supporting cardiac revascularization before major surgery has been lacking. The study investigators sought to clarify this uncertainty.
The prospective trial enrolled 510 patients at 18 VA centers. Patients scheduled for major vascular operations were eligible, and were preoperatively assessed via clinical criteria, stress imaging, and angiography when appropriate. Eligible patients had significant (at least 70%) stenosis of at least one coronary artery. High-risk patients (i.e., those with left main disease, severe aortic stenosis, and LVEF <20%) were excluded. Patients were then randomized to one
of two groups. The first group underwent revascularization with PTCA or CABG plus medical optimization; the second group received only medical optimization. Most patients in both groups received beta-blockers, and more than half in each received statins. The patient populations were appropriately randomized, although overwhelmingly male (98%). Most patients had one- or two-vessel CAD. The primary endpoint was long-term mortality. Secondary endpoints included MI, stroke, renal failure requiring dialysis, and limb loss. Follow-up rates were similar in both groups (86% and 85%).
The major finding of the study was the lack of difference in mortality between the two groups at an average follow-up of 2.7 years (22% vs. 23%, RR= 0.98, 95% CI 0.70 to 1.37, p = 0.92). Analyzing by “treatment-received” instead of “intention-to-treat” did not significantly change this result. Of note, ten patients in the revascularization arm died between the revascularization procedure and the vascular surgery. Not surprisingly, revascularization also delayed the time to surgery for patients in that arm of the study. In the authors’ analysis, the patients were also divided into subgroups based on high-risk variables (prior CABG, category of Revised Cardiac Risk Index, etc.), but the study was not powered to detect mortality differences between the two arms within these subgroups. The authors concluded that there was no benefit to revascularization in patients with stable coronary syndromes prior to elective vascular surgeries.
The results of this study validate the conservative practice recommended by the existing guidelines— that is, to perform revascularization procedures in the preoperative setting only when indicated by clinical criteria such as unstable ischemic symptoms, and if likely to improve long-term survival. Beta-blockers, and based on recent studies probably “statins,” should continue to be the mainstay of perioperative risk optimization for patients with stable coronary disease.
There were, however, several important considerations: first, the study group was exclusively male, although there is little reason to believe that women would have better outcomes from revascularization. And second, the highest-risk patients were excluded, and therefore the results should not be extrapolated to that population. Prospective identification of the group of patients who may benefit from aggressive intervention should remain a target of risk assessment and further research. (BH)
Blood Transfusion May Increase Mortality in Acute Coronary Syndrome
Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555-62.
The increased use of invasive procedures and anticoagulant and fibrinolytic drugs in patients with ischemic heart disease in recent years predictably increases the potential for bleeding and perceived need for transfusion. Studies evaluating the association between transfusion and mortality have produced mixed results. A more pertinent clinical question is whether transfusion is beneficial or harmful in patients with acute coronary syndromes who acutely develop anemia during their hospitalization.
The authors used clinical data from three large international trials of patients with acute coronary syndromes (GUSTO IIb, PURSUIT, and PARAGON B) to determine the association between blood transfusion and outcomes among patients who developed moderate to severe bleeding, anemia, or both during their hospitalization.
Assessment of clinically significant bleeding complications was based on the GUSTO definition of severe (intracranial hemorrhage or hemodynamic compromise and requiring intervention) or moderate (hemodynamically stable but requiring blood transfusion) bleeding. The GUSTO IIb and PURSUIT trials used the above definition; PARAGON B categorized bleeding as “major or life threatening” (intracranial hemorrhage or bleeding leading to hemodynamic compromise requiring intervention) or “intermediate” (requiring transfusion or a decrease in hemoglobin of 5 g/dL or more, or a decrease in hematocrit ( 15%). Major or life-threatening bleeding episodes and intermediate bleeding episodes in PARAGON B were deemed equivalent to severe and moderate bleeding episodes in GUSTO.
Data were collected on the date, time, severity, and location of each bleeding event, and on the date and number of units of packed red blood cells and whole blood transfused. The primary end-point was 30-day all-cause mortality. Secondary end-points were occurrence of the composite of 30-day death or MI.
The unadjusted rates of 30-day death, MI, and composite death/MI were significantly higher among patients who received a transfusion (30-day death, 8.00% vs. 3.08%; p<.001; 30-day MI, 25.16% vs. 8.16%; p<.001; 30-day composite death/MI, 29.24% vs. 10.02%; p<.001).
After adjustment for baseline characteristics, bleeding and transfusion propensity, and nadir hematocrit, blood transfusion was associated with a hazard ratio for death of 3.94 (95% confidence interval, 3.26–4.75).
No significant association was found between transfusion and 30-day mortality at a nadir hematocrit of 25% or less (adjusted OR 1.13; 95% CI 0.70-1.82). However, at a nadir hematocrit higher than 25%, transfusion was associated with significantly higher odds of 30-day death, even after excluding patients who underwent CABG or those who died within the first 5 days of follow-up.
These findings differ from the findings of Wu et al. (1) who noted that blood transfusion was associated with lower 30-day mortality among elderly patients with MI if the admission hematocrit was 30% or lower. The current authors propose that their data is more robust due to meticulous collection through clinical trial records, and that their analysis accounts for timing of transfusion and indications for transfusion.
Many clinicians logically believe that augmentation of oxygen carrying capacity via transfusion would be beneficial to patients with active ischemia. However, the authors note that red blood cells in stored blood may be depleted of both 2,3-diphosphoglyceric acid and nitric oxide, both of which are critical components to oxygen delivery and exchange. These cells then function as nitric oxide “sinks,” promoting vasoconstriction, platelet aggregation, and impaired oxygen delivery to tissues. In addition, inflammatory mediators associated with exacerbation of myocardial ischemia may remain in transfused blood, potentially contributing to adverse outcomes.
As this is a nonrandomized, post hoc observational study, further prescriptive conclusions regarding transfusion cannot be made. However, the authors, along with an accompanying editorial, call for prospective randomized trials of transfusion in anemic patients with acute coronary syndromes to better define the role of this commonly used therapy. (CW)
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230-6.
Cost-effectiveness of Rhythm Versus Rate Control in Atrial Fibrillation
Marshall DA, Levy AR, Vidaillet H, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Intern Med. 2004;141:653-61.
Atrial fibrillation is the most common arrhythmia treated by physicians. It afflicts nearly 10% of patients age 80 years or older. There are two primary modalities of managing patients with atrial fibrillation; rate control versus cardioversion into sinus rhythm.
AFFIRM was a multicenter randomized controlled trial involving 213 centers in USA and Canada that compared rate versus rhythm control in 4060 patients. These patients had a mean age of 70 years. Sixty-one percent of the enrolled patients were men, and 66% had recurrent atrial fibrillation. Seventy-one percent of patients had hypertension, 39% had coronary artery disease, and 9% had congestive heart failure. Patients were block randomized by center to either rate control or rhythm control and followed for an average of 3.5 years. AFFIRM results showed no significant mortality difference between the two groups (hazard ratio for rate versus rhythm control, 0.87 with 95% CI, 0.75 to 1.01).
Primary data on survival and resource utilization were used to conduct the economic analysis from a third party payer perspective. Authors used intention to treat data for the economic analysis.
For resource utilization estimates, US healthcare cost figures for the year 2002 were used. All earlier costs were appropriately adjusted using Consumer Price Index, Medical Care component to estimate their nominal values in year 2002. Hospital costs were taken as the mean charges per day from Healthcare Cost and Utilization Project statistics for Diseases of the Circulatory System for patients older than 18 years age. Low and high end of these costs were assumed to be equivalent to 25th and 75th percentiles of the mean costs, respectively. Physician costs were assumed to be equivalent to be the average of all carriers’ payments for the relative value units of the services rendered based on a generic current procedural terminology code. Sensitivity analysis was conducted on these physician costs using minimum payment among these carriers as the low cost estimate and the standard charges for Marshfield Clinic for the high end. Costs of pacemaker and implantable cardioverter defibrillators were based on manufacturers’ list prices. For sensitivity analysis, hardware costs were excluded for low cost estimates and the maximum manufacturers’ list price for the high estimate was used.
At each follow-up visit during the AFFIRM trial, the number of cardioversion attempts since the prior visit was recorded. Costs of cardioversion were based on average payment to Marshfield Clinic for outpatient electrical cardioversion for the year 2002. Authors assigned no costs for low cost estimate and used billed charges for high costs for sensitivity analysis.
At each follow-up visit, the number of short stay and emergency department visits since the prior visit was recorded. Weighted average Medicare costs for level I and II facilities were used as the baseline estimate for these visits. Estimates for sensitivity analysis were the minimum and maximum Medicare payments for these visits. Physician fees were based on level III emergency department visit with low and high cost estimates assigned as described above.
Medication costs were based on the least average wholesale price (AWP) for a generic medication. Low and high cost estimates were taken from lowest quoted US Internet pharmacy price and highest AWP for the most expensive drug in the class respectively. Only medications used for atrial fibrillation and anticoagulation were considered for analysis.
The authors calculated the mean cost per patient In the Literature (continued) and the mean survival time between the two interventions. Future costs were discounted by 3%. For the base estimate, rhythm control was more expensive and less effective than rate control, i.e., dominated by the rate control. Rate control dominated rhythm control even for high and low estimates of the sensitivity analysis demonstrating stability of the results. Authors used 10,000 simulations to perform non-parametric bootstrapping analysis to find the 95% credible intervals around the base estimate. The bootstrap results showed that for 95% of the results rate control had higher survival time and was less costly than rhythm control. These simulation results clearly showed rate control is more cost-effective for patient population resembling that of AFFIRM trial.
The study has some limitations. These results are robust for patients similar to those in AFFIRM trial, i.e., older patients with cardiovascular defects that are at risk of cerebrovascular embolism. However these results may not be applicable to younger patients and those with “lone atrial fibrillation.” The study had a follow-up period of 3.5 years, and the cost-effectiveness analysis is confined to this period. It is difficult to determine mortality advantage of one treatment over another within the limited duration of the AFFIRM study. Most of the patients were on multiple pharmacologic agents for rhythm control and had a high incidence of cross-over from rhythm control to rate control reflecting modest benefits of the current agents. These results may not be applicable to patients whose atrial fibrillation is well controlled by a single agent or by non-pharmacological treatment. Patients on rhythm control agents had, as expected, more hospitalization days from the side effects and treatment protocols of the agents (especially pharmacologic) used to control the rhythm. With advances in both pharmacologic as well as nonpharmacologic methods for rhythm control generating safer and more efficacious technologies, the results of this analysis may become less valid in the future. The analysis was conducted from a third-party payer perspective, without accounting for the quality of life. Thus patients who have symptomatic atrial fibrillation and those with diastolic dysfunction may have improved quality of life from rhythm control over just rate control. The results may not be applicable to these patients. (SS)
CARP Trial Suggests No Benefit to Revascularization Before Vascular Surgery
McFalls, EO, Ward HB, Mortiz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
Recent studies have presented evidence that treatment with beta-blockers for patients with CAD could reduce the risk of perioperative cardiac complications. Beta-blockers have since become a critical part of the management plan for the perioperative patient. Evidence-based practice guidelines for cardiac risk assessment have been published by both the American College of Physicians and the American College of Cardiology/American Heart Association. However, practice patterns continue to vary between physicians and cardiologists, particularly for patients clinically stratified into the intermediate-risk category. Some physicians feel comfortable with a conservative approach of medical optimization even in the setting of established CAD, while others favor more aggressive treatment, even though the prospective data supporting cardiac revascularization before major surgery has been lacking. The study investigators sought to clarify this uncertainty.
The prospective trial enrolled 510 patients at 18 VA centers. Patients scheduled for major vascular operations were eligible, and were preoperatively assessed via clinical criteria, stress imaging, and angiography when appropriate. Eligible patients had significant (at least 70%) stenosis of at least one coronary artery. High-risk patients (i.e., those with left main disease, severe aortic stenosis, and LVEF <20%) were excluded. Patients were then randomized to one
of two groups. The first group underwent revascularization with PTCA or CABG plus medical optimization; the second group received only medical optimization. Most patients in both groups received beta-blockers, and more than half in each received statins. The patient populations were appropriately randomized, although overwhelmingly male (98%). Most patients had one- or two-vessel CAD. The primary endpoint was long-term mortality. Secondary endpoints included MI, stroke, renal failure requiring dialysis, and limb loss. Follow-up rates were similar in both groups (86% and 85%).
The major finding of the study was the lack of difference in mortality between the two groups at an average follow-up of 2.7 years (22% vs. 23%, RR= 0.98, 95% CI 0.70 to 1.37, p = 0.92). Analyzing by “treatment-received” instead of “intention-to-treat” did not significantly change this result. Of note, ten patients in the revascularization arm died between the revascularization procedure and the vascular surgery. Not surprisingly, revascularization also delayed the time to surgery for patients in that arm of the study. In the authors’ analysis, the patients were also divided into subgroups based on high-risk variables (prior CABG, category of Revised Cardiac Risk Index, etc.), but the study was not powered to detect mortality differences between the two arms within these subgroups. The authors concluded that there was no benefit to revascularization in patients with stable coronary syndromes prior to elective vascular surgeries.
The results of this study validate the conservative practice recommended by the existing guidelines— that is, to perform revascularization procedures in the preoperative setting only when indicated by clinical criteria such as unstable ischemic symptoms, and if likely to improve long-term survival. Beta-blockers, and based on recent studies probably “statins,” should continue to be the mainstay of perioperative risk optimization for patients with stable coronary disease.
There were, however, several important considerations: first, the study group was exclusively male, although there is little reason to believe that women would have better outcomes from revascularization. And second, the highest-risk patients were excluded, and therefore the results should not be extrapolated to that population. Prospective identification of the group of patients who may benefit from aggressive intervention should remain a target of risk assessment and further research. (BH)
Blood Transfusion May Increase Mortality in Acute Coronary Syndrome
Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555-62.
The increased use of invasive procedures and anticoagulant and fibrinolytic drugs in patients with ischemic heart disease in recent years predictably increases the potential for bleeding and perceived need for transfusion. Studies evaluating the association between transfusion and mortality have produced mixed results. A more pertinent clinical question is whether transfusion is beneficial or harmful in patients with acute coronary syndromes who acutely develop anemia during their hospitalization.
The authors used clinical data from three large international trials of patients with acute coronary syndromes (GUSTO IIb, PURSUIT, and PARAGON B) to determine the association between blood transfusion and outcomes among patients who developed moderate to severe bleeding, anemia, or both during their hospitalization.
Assessment of clinically significant bleeding complications was based on the GUSTO definition of severe (intracranial hemorrhage or hemodynamic compromise and requiring intervention) or moderate (hemodynamically stable but requiring blood transfusion) bleeding. The GUSTO IIb and PURSUIT trials used the above definition; PARAGON B categorized bleeding as “major or life threatening” (intracranial hemorrhage or bleeding leading to hemodynamic compromise requiring intervention) or “intermediate” (requiring transfusion or a decrease in hemoglobin of 5 g/dL or more, or a decrease in hematocrit ( 15%). Major or life-threatening bleeding episodes and intermediate bleeding episodes in PARAGON B were deemed equivalent to severe and moderate bleeding episodes in GUSTO.
Data were collected on the date, time, severity, and location of each bleeding event, and on the date and number of units of packed red blood cells and whole blood transfused. The primary end-point was 30-day all-cause mortality. Secondary end-points were occurrence of the composite of 30-day death or MI.
The unadjusted rates of 30-day death, MI, and composite death/MI were significantly higher among patients who received a transfusion (30-day death, 8.00% vs. 3.08%; p<.001; 30-day MI, 25.16% vs. 8.16%; p<.001; 30-day composite death/MI, 29.24% vs. 10.02%; p<.001).
After adjustment for baseline characteristics, bleeding and transfusion propensity, and nadir hematocrit, blood transfusion was associated with a hazard ratio for death of 3.94 (95% confidence interval, 3.26–4.75).
No significant association was found between transfusion and 30-day mortality at a nadir hematocrit of 25% or less (adjusted OR 1.13; 95% CI 0.70-1.82). However, at a nadir hematocrit higher than 25%, transfusion was associated with significantly higher odds of 30-day death, even after excluding patients who underwent CABG or those who died within the first 5 days of follow-up.
These findings differ from the findings of Wu et al. (1) who noted that blood transfusion was associated with lower 30-day mortality among elderly patients with MI if the admission hematocrit was 30% or lower. The current authors propose that their data is more robust due to meticulous collection through clinical trial records, and that their analysis accounts for timing of transfusion and indications for transfusion.
Many clinicians logically believe that augmentation of oxygen carrying capacity via transfusion would be beneficial to patients with active ischemia. However, the authors note that red blood cells in stored blood may be depleted of both 2,3-diphosphoglyceric acid and nitric oxide, both of which are critical components to oxygen delivery and exchange. These cells then function as nitric oxide “sinks,” promoting vasoconstriction, platelet aggregation, and impaired oxygen delivery to tissues. In addition, inflammatory mediators associated with exacerbation of myocardial ischemia may remain in transfused blood, potentially contributing to adverse outcomes.
As this is a nonrandomized, post hoc observational study, further prescriptive conclusions regarding transfusion cannot be made. However, the authors, along with an accompanying editorial, call for prospective randomized trials of transfusion in anemic patients with acute coronary syndromes to better define the role of this commonly used therapy. (CW)
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230-6.
Cost-effectiveness of Rhythm Versus Rate Control in Atrial Fibrillation
Marshall DA, Levy AR, Vidaillet H, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Intern Med. 2004;141:653-61.
Atrial fibrillation is the most common arrhythmia treated by physicians. It afflicts nearly 10% of patients age 80 years or older. There are two primary modalities of managing patients with atrial fibrillation; rate control versus cardioversion into sinus rhythm.
AFFIRM was a multicenter randomized controlled trial involving 213 centers in USA and Canada that compared rate versus rhythm control in 4060 patients. These patients had a mean age of 70 years. Sixty-one percent of the enrolled patients were men, and 66% had recurrent atrial fibrillation. Seventy-one percent of patients had hypertension, 39% had coronary artery disease, and 9% had congestive heart failure. Patients were block randomized by center to either rate control or rhythm control and followed for an average of 3.5 years. AFFIRM results showed no significant mortality difference between the two groups (hazard ratio for rate versus rhythm control, 0.87 with 95% CI, 0.75 to 1.01).
Primary data on survival and resource utilization were used to conduct the economic analysis from a third party payer perspective. Authors used intention to treat data for the economic analysis.
For resource utilization estimates, US healthcare cost figures for the year 2002 were used. All earlier costs were appropriately adjusted using Consumer Price Index, Medical Care component to estimate their nominal values in year 2002. Hospital costs were taken as the mean charges per day from Healthcare Cost and Utilization Project statistics for Diseases of the Circulatory System for patients older than 18 years age. Low and high end of these costs were assumed to be equivalent to 25th and 75th percentiles of the mean costs, respectively. Physician costs were assumed to be equivalent to be the average of all carriers’ payments for the relative value units of the services rendered based on a generic current procedural terminology code. Sensitivity analysis was conducted on these physician costs using minimum payment among these carriers as the low cost estimate and the standard charges for Marshfield Clinic for the high end. Costs of pacemaker and implantable cardioverter defibrillators were based on manufacturers’ list prices. For sensitivity analysis, hardware costs were excluded for low cost estimates and the maximum manufacturers’ list price for the high estimate was used.
At each follow-up visit during the AFFIRM trial, the number of cardioversion attempts since the prior visit was recorded. Costs of cardioversion were based on average payment to Marshfield Clinic for outpatient electrical cardioversion for the year 2002. Authors assigned no costs for low cost estimate and used billed charges for high costs for sensitivity analysis.
At each follow-up visit, the number of short stay and emergency department visits since the prior visit was recorded. Weighted average Medicare costs for level I and II facilities were used as the baseline estimate for these visits. Estimates for sensitivity analysis were the minimum and maximum Medicare payments for these visits. Physician fees were based on level III emergency department visit with low and high cost estimates assigned as described above.
Medication costs were based on the least average wholesale price (AWP) for a generic medication. Low and high cost estimates were taken from lowest quoted US Internet pharmacy price and highest AWP for the most expensive drug in the class respectively. Only medications used for atrial fibrillation and anticoagulation were considered for analysis.
The authors calculated the mean cost per patient In the Literature (continued) and the mean survival time between the two interventions. Future costs were discounted by 3%. For the base estimate, rhythm control was more expensive and less effective than rate control, i.e., dominated by the rate control. Rate control dominated rhythm control even for high and low estimates of the sensitivity analysis demonstrating stability of the results. Authors used 10,000 simulations to perform non-parametric bootstrapping analysis to find the 95% credible intervals around the base estimate. The bootstrap results showed that for 95% of the results rate control had higher survival time and was less costly than rhythm control. These simulation results clearly showed rate control is more cost-effective for patient population resembling that of AFFIRM trial.
The study has some limitations. These results are robust for patients similar to those in AFFIRM trial, i.e., older patients with cardiovascular defects that are at risk of cerebrovascular embolism. However these results may not be applicable to younger patients and those with “lone atrial fibrillation.” The study had a follow-up period of 3.5 years, and the cost-effectiveness analysis is confined to this period. It is difficult to determine mortality advantage of one treatment over another within the limited duration of the AFFIRM study. Most of the patients were on multiple pharmacologic agents for rhythm control and had a high incidence of cross-over from rhythm control to rate control reflecting modest benefits of the current agents. These results may not be applicable to patients whose atrial fibrillation is well controlled by a single agent or by non-pharmacological treatment. Patients on rhythm control agents had, as expected, more hospitalization days from the side effects and treatment protocols of the agents (especially pharmacologic) used to control the rhythm. With advances in both pharmacologic as well as nonpharmacologic methods for rhythm control generating safer and more efficacious technologies, the results of this analysis may become less valid in the future. The analysis was conducted from a third-party payer perspective, without accounting for the quality of life. Thus patients who have symptomatic atrial fibrillation and those with diastolic dysfunction may have improved quality of life from rhythm control over just rate control. The results may not be applicable to these patients. (SS)
CARP Trial Suggests No Benefit to Revascularization Before Vascular Surgery
McFalls, EO, Ward HB, Mortiz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
Recent studies have presented evidence that treatment with beta-blockers for patients with CAD could reduce the risk of perioperative cardiac complications. Beta-blockers have since become a critical part of the management plan for the perioperative patient. Evidence-based practice guidelines for cardiac risk assessment have been published by both the American College of Physicians and the American College of Cardiology/American Heart Association. However, practice patterns continue to vary between physicians and cardiologists, particularly for patients clinically stratified into the intermediate-risk category. Some physicians feel comfortable with a conservative approach of medical optimization even in the setting of established CAD, while others favor more aggressive treatment, even though the prospective data supporting cardiac revascularization before major surgery has been lacking. The study investigators sought to clarify this uncertainty.
The prospective trial enrolled 510 patients at 18 VA centers. Patients scheduled for major vascular operations were eligible, and were preoperatively assessed via clinical criteria, stress imaging, and angiography when appropriate. Eligible patients had significant (at least 70%) stenosis of at least one coronary artery. High-risk patients (i.e., those with left main disease, severe aortic stenosis, and LVEF <20%) were excluded. Patients were then randomized to one
of two groups. The first group underwent revascularization with PTCA or CABG plus medical optimization; the second group received only medical optimization. Most patients in both groups received beta-blockers, and more than half in each received statins. The patient populations were appropriately randomized, although overwhelmingly male (98%). Most patients had one- or two-vessel CAD. The primary endpoint was long-term mortality. Secondary endpoints included MI, stroke, renal failure requiring dialysis, and limb loss. Follow-up rates were similar in both groups (86% and 85%).
The major finding of the study was the lack of difference in mortality between the two groups at an average follow-up of 2.7 years (22% vs. 23%, RR= 0.98, 95% CI 0.70 to 1.37, p = 0.92). Analyzing by “treatment-received” instead of “intention-to-treat” did not significantly change this result. Of note, ten patients in the revascularization arm died between the revascularization procedure and the vascular surgery. Not surprisingly, revascularization also delayed the time to surgery for patients in that arm of the study. In the authors’ analysis, the patients were also divided into subgroups based on high-risk variables (prior CABG, category of Revised Cardiac Risk Index, etc.), but the study was not powered to detect mortality differences between the two arms within these subgroups. The authors concluded that there was no benefit to revascularization in patients with stable coronary syndromes prior to elective vascular surgeries.
The results of this study validate the conservative practice recommended by the existing guidelines— that is, to perform revascularization procedures in the preoperative setting only when indicated by clinical criteria such as unstable ischemic symptoms, and if likely to improve long-term survival. Beta-blockers, and based on recent studies probably “statins,” should continue to be the mainstay of perioperative risk optimization for patients with stable coronary disease.
There were, however, several important considerations: first, the study group was exclusively male, although there is little reason to believe that women would have better outcomes from revascularization. And second, the highest-risk patients were excluded, and therefore the results should not be extrapolated to that population. Prospective identification of the group of patients who may benefit from aggressive intervention should remain a target of risk assessment and further research. (BH)
Blood Transfusion May Increase Mortality in Acute Coronary Syndrome
Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555-62.
The increased use of invasive procedures and anticoagulant and fibrinolytic drugs in patients with ischemic heart disease in recent years predictably increases the potential for bleeding and perceived need for transfusion. Studies evaluating the association between transfusion and mortality have produced mixed results. A more pertinent clinical question is whether transfusion is beneficial or harmful in patients with acute coronary syndromes who acutely develop anemia during their hospitalization.
The authors used clinical data from three large international trials of patients with acute coronary syndromes (GUSTO IIb, PURSUIT, and PARAGON B) to determine the association between blood transfusion and outcomes among patients who developed moderate to severe bleeding, anemia, or both during their hospitalization.
Assessment of clinically significant bleeding complications was based on the GUSTO definition of severe (intracranial hemorrhage or hemodynamic compromise and requiring intervention) or moderate (hemodynamically stable but requiring blood transfusion) bleeding. The GUSTO IIb and PURSUIT trials used the above definition; PARAGON B categorized bleeding as “major or life threatening” (intracranial hemorrhage or bleeding leading to hemodynamic compromise requiring intervention) or “intermediate” (requiring transfusion or a decrease in hemoglobin of 5 g/dL or more, or a decrease in hematocrit ( 15%). Major or life-threatening bleeding episodes and intermediate bleeding episodes in PARAGON B were deemed equivalent to severe and moderate bleeding episodes in GUSTO.
Data were collected on the date, time, severity, and location of each bleeding event, and on the date and number of units of packed red blood cells and whole blood transfused. The primary end-point was 30-day all-cause mortality. Secondary end-points were occurrence of the composite of 30-day death or MI.
The unadjusted rates of 30-day death, MI, and composite death/MI were significantly higher among patients who received a transfusion (30-day death, 8.00% vs. 3.08%; p<.001; 30-day MI, 25.16% vs. 8.16%; p<.001; 30-day composite death/MI, 29.24% vs. 10.02%; p<.001).
After adjustment for baseline characteristics, bleeding and transfusion propensity, and nadir hematocrit, blood transfusion was associated with a hazard ratio for death of 3.94 (95% confidence interval, 3.26–4.75).
No significant association was found between transfusion and 30-day mortality at a nadir hematocrit of 25% or less (adjusted OR 1.13; 95% CI 0.70-1.82). However, at a nadir hematocrit higher than 25%, transfusion was associated with significantly higher odds of 30-day death, even after excluding patients who underwent CABG or those who died within the first 5 days of follow-up.
These findings differ from the findings of Wu et al. (1) who noted that blood transfusion was associated with lower 30-day mortality among elderly patients with MI if the admission hematocrit was 30% or lower. The current authors propose that their data is more robust due to meticulous collection through clinical trial records, and that their analysis accounts for timing of transfusion and indications for transfusion.
Many clinicians logically believe that augmentation of oxygen carrying capacity via transfusion would be beneficial to patients with active ischemia. However, the authors note that red blood cells in stored blood may be depleted of both 2,3-diphosphoglyceric acid and nitric oxide, both of which are critical components to oxygen delivery and exchange. These cells then function as nitric oxide “sinks,” promoting vasoconstriction, platelet aggregation, and impaired oxygen delivery to tissues. In addition, inflammatory mediators associated with exacerbation of myocardial ischemia may remain in transfused blood, potentially contributing to adverse outcomes.
As this is a nonrandomized, post hoc observational study, further prescriptive conclusions regarding transfusion cannot be made. However, the authors, along with an accompanying editorial, call for prospective randomized trials of transfusion in anemic patients with acute coronary syndromes to better define the role of this commonly used therapy. (CW)
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230-6.
Cost-effectiveness of Rhythm Versus Rate Control in Atrial Fibrillation
Marshall DA, Levy AR, Vidaillet H, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Intern Med. 2004;141:653-61.
Atrial fibrillation is the most common arrhythmia treated by physicians. It afflicts nearly 10% of patients age 80 years or older. There are two primary modalities of managing patients with atrial fibrillation; rate control versus cardioversion into sinus rhythm.
AFFIRM was a multicenter randomized controlled trial involving 213 centers in USA and Canada that compared rate versus rhythm control in 4060 patients. These patients had a mean age of 70 years. Sixty-one percent of the enrolled patients were men, and 66% had recurrent atrial fibrillation. Seventy-one percent of patients had hypertension, 39% had coronary artery disease, and 9% had congestive heart failure. Patients were block randomized by center to either rate control or rhythm control and followed for an average of 3.5 years. AFFIRM results showed no significant mortality difference between the two groups (hazard ratio for rate versus rhythm control, 0.87 with 95% CI, 0.75 to 1.01).
Primary data on survival and resource utilization were used to conduct the economic analysis from a third party payer perspective. Authors used intention to treat data for the economic analysis.
For resource utilization estimates, US healthcare cost figures for the year 2002 were used. All earlier costs were appropriately adjusted using Consumer Price Index, Medical Care component to estimate their nominal values in year 2002. Hospital costs were taken as the mean charges per day from Healthcare Cost and Utilization Project statistics for Diseases of the Circulatory System for patients older than 18 years age. Low and high end of these costs were assumed to be equivalent to 25th and 75th percentiles of the mean costs, respectively. Physician costs were assumed to be equivalent to be the average of all carriers’ payments for the relative value units of the services rendered based on a generic current procedural terminology code. Sensitivity analysis was conducted on these physician costs using minimum payment among these carriers as the low cost estimate and the standard charges for Marshfield Clinic for the high end. Costs of pacemaker and implantable cardioverter defibrillators were based on manufacturers’ list prices. For sensitivity analysis, hardware costs were excluded for low cost estimates and the maximum manufacturers’ list price for the high estimate was used.
At each follow-up visit during the AFFIRM trial, the number of cardioversion attempts since the prior visit was recorded. Costs of cardioversion were based on average payment to Marshfield Clinic for outpatient electrical cardioversion for the year 2002. Authors assigned no costs for low cost estimate and used billed charges for high costs for sensitivity analysis.
At each follow-up visit, the number of short stay and emergency department visits since the prior visit was recorded. Weighted average Medicare costs for level I and II facilities were used as the baseline estimate for these visits. Estimates for sensitivity analysis were the minimum and maximum Medicare payments for these visits. Physician fees were based on level III emergency department visit with low and high cost estimates assigned as described above.
Medication costs were based on the least average wholesale price (AWP) for a generic medication. Low and high cost estimates were taken from lowest quoted US Internet pharmacy price and highest AWP for the most expensive drug in the class respectively. Only medications used for atrial fibrillation and anticoagulation were considered for analysis.
The authors calculated the mean cost per patient In the Literature (continued) and the mean survival time between the two interventions. Future costs were discounted by 3%. For the base estimate, rhythm control was more expensive and less effective than rate control, i.e., dominated by the rate control. Rate control dominated rhythm control even for high and low estimates of the sensitivity analysis demonstrating stability of the results. Authors used 10,000 simulations to perform non-parametric bootstrapping analysis to find the 95% credible intervals around the base estimate. The bootstrap results showed that for 95% of the results rate control had higher survival time and was less costly than rhythm control. These simulation results clearly showed rate control is more cost-effective for patient population resembling that of AFFIRM trial.
The study has some limitations. These results are robust for patients similar to those in AFFIRM trial, i.e., older patients with cardiovascular defects that are at risk of cerebrovascular embolism. However these results may not be applicable to younger patients and those with “lone atrial fibrillation.” The study had a follow-up period of 3.5 years, and the cost-effectiveness analysis is confined to this period. It is difficult to determine mortality advantage of one treatment over another within the limited duration of the AFFIRM study. Most of the patients were on multiple pharmacologic agents for rhythm control and had a high incidence of cross-over from rhythm control to rate control reflecting modest benefits of the current agents. These results may not be applicable to patients whose atrial fibrillation is well controlled by a single agent or by non-pharmacological treatment. Patients on rhythm control agents had, as expected, more hospitalization days from the side effects and treatment protocols of the agents (especially pharmacologic) used to control the rhythm. With advances in both pharmacologic as well as nonpharmacologic methods for rhythm control generating safer and more efficacious technologies, the results of this analysis may become less valid in the future. The analysis was conducted from a third-party payer perspective, without accounting for the quality of life. Thus patients who have symptomatic atrial fibrillation and those with diastolic dysfunction may have improved quality of life from rhythm control over just rate control. The results may not be applicable to these patients. (SS)