User login
Clinical Progress Note: Myocardial Injury After Noncardiac Surgery
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
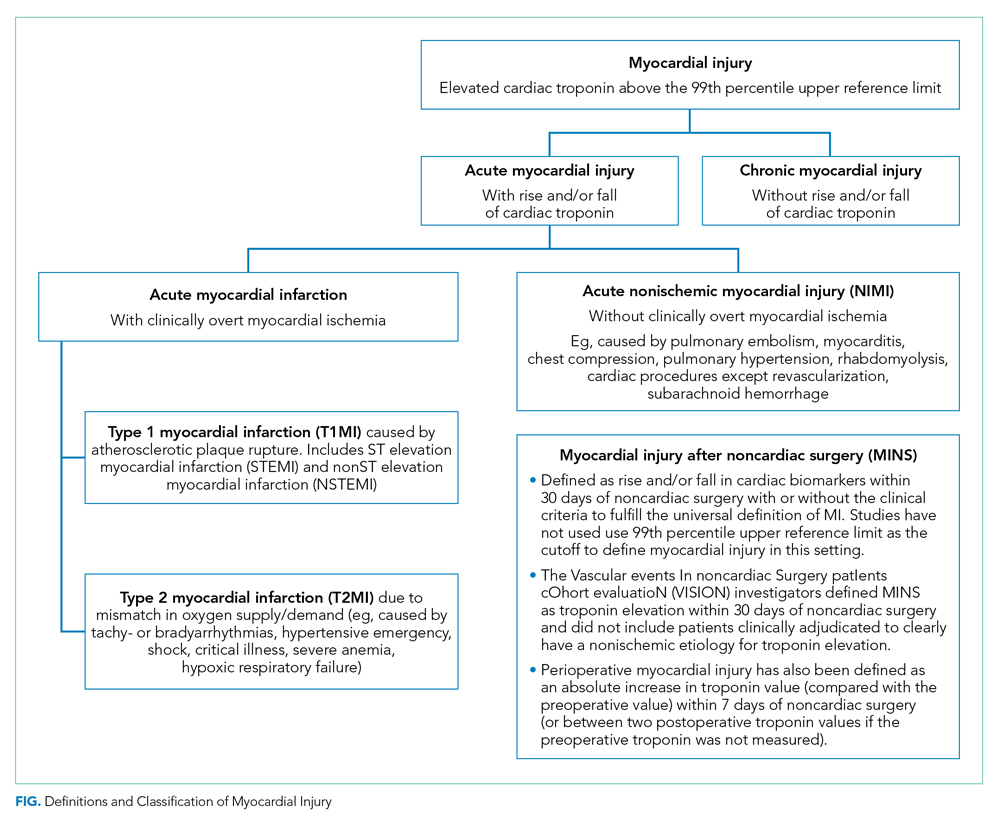
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8
EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
© 2020 Society of Hospital Medicine