User login
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
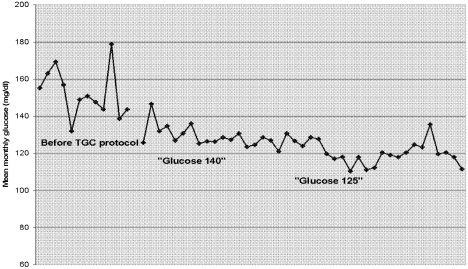
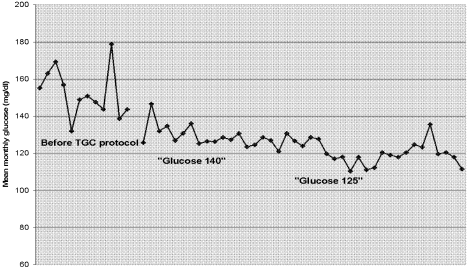
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.
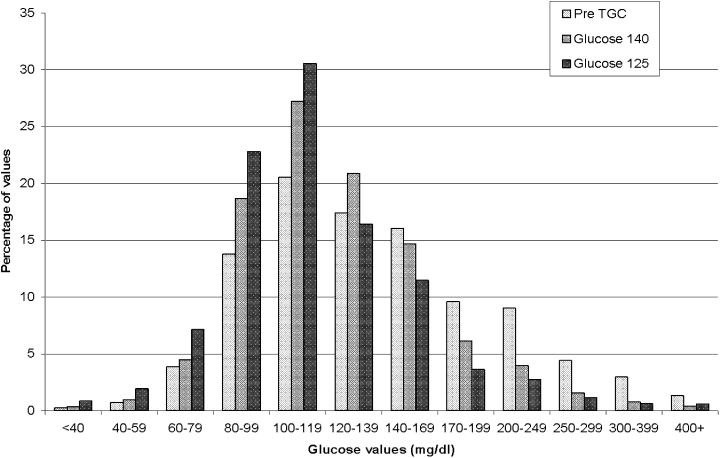
Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.
The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
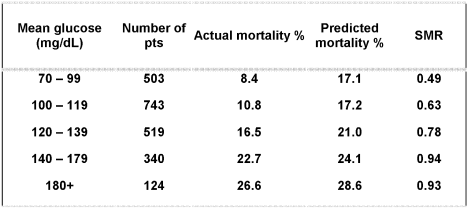
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.
Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
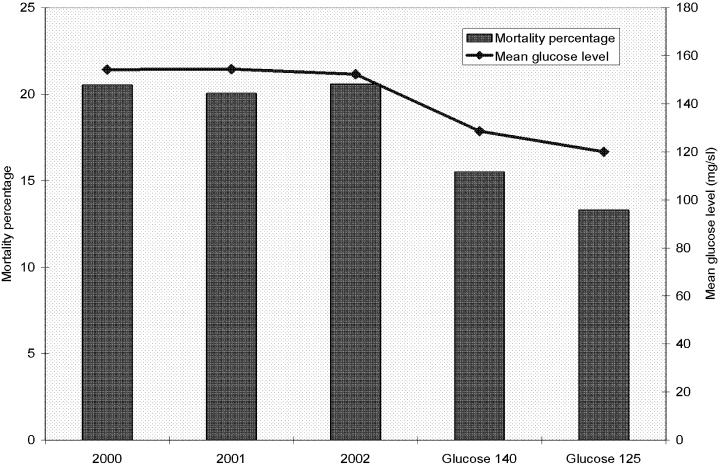
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.
Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.

Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.

The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.

Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.

Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.

Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.

The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.

Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.

Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.